User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=ß-catenin= | =ß-catenin= | ||
| - | ß-catenin is an important element in cell adherens junctions | + | |
| + | ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota phylum, in humans the gene CTNNB1 transcribes a 95kDa protein that allow cadherins to anchor in intracellular cytoeskeleton (actin) by connection cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway (https://www.annualreviews.org/doi/full/10.1146/annurev.cellbio.20.010403.113126), related to embryonic development. Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> | ||
<StructureSection load='2Z6G' size='400' caption='Structure of ß-catenin from Zebrafish' scene='84/848919/Dotsbetacateninacoloridaartigo/1'> | <StructureSection load='2Z6G' size='400' caption='Structure of ß-catenin from Zebrafish' scene='84/848919/Dotsbetacateninacoloridaartigo/1'> | ||
| Line 11: | Line 12: | ||
The terminal domains sequences are less conserved than the armadillo repeat domain, mediate some of the protein interactions and are both negatively charged. It is observed that the <scene name='84/848919/C-helix3correta/1'>helix-C constitutes the C-terminal domain</scene>, and the N terminus of the first armadillo repeat has an <scene name='84/848919/Correton-terminushelix/1'>extra alpha helix</scene>. Both N- and C-terminal domains do not interact with the armadillo repeat domain. <ref name="xing2009" /> | The terminal domains sequences are less conserved than the armadillo repeat domain, mediate some of the protein interactions and are both negatively charged. It is observed that the <scene name='84/848919/C-helix3correta/1'>helix-C constitutes the C-terminal domain</scene>, and the N terminus of the first armadillo repeat has an <scene name='84/848919/Correton-terminushelix/1'>extra alpha helix</scene>. Both N- and C-terminal domains do not interact with the armadillo repeat domain. <ref name="xing2009" /> | ||
| - | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The | + | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Hydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats.</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues. Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. <ref name="xing2009" /> |
| - | It is possible that the | + | It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> |
==Cell Adhesion== | ==Cell Adhesion== | ||
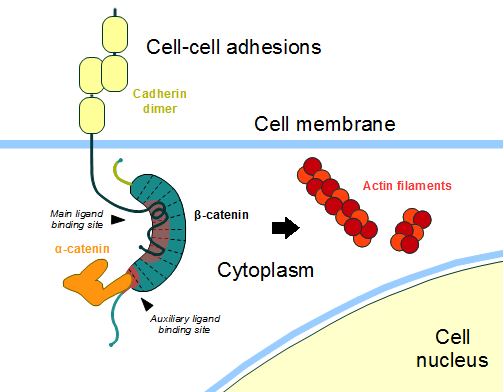
| - | In the absence of Wnt stimulus, the ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent | + | In the absence of Wnt stimulus, the ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin (but alpha-catenin, p120-catenin too) by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted, in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT). <ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> |
The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X]) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X]) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | ||
| Line 24: | Line 25: | ||
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
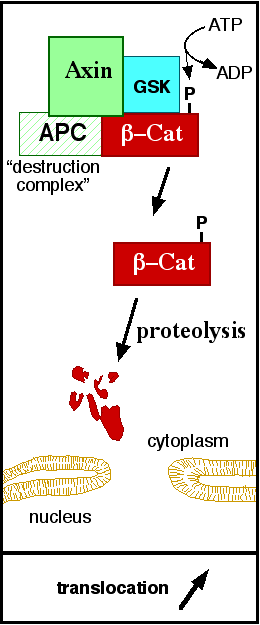
| - | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 2), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. <ref name="valenta2012" /> | + | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 2), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. Besides that, the relation Wnt/ß-catenin pathway usually are reported by 'canonical' and 'non-canonical', whose meaning remotes to the components of the cascate. The first, leads to accumulation and stabilization cytosolic (unphosphorylated) ß-catenin and the second, promotes the increase wheter in intracellular calcium or mediate cell polarity, but both are established in embryonic development of normal tissue and organs as mentioned before<ref name="valenta2012" /> |
[[Image:Axindestructioncomplex.png]] | [[Image:Axindestructioncomplex.png]] | ||
Revision as of 23:10, 21 June 2020
ß-catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota phylum, in humans the gene CTNNB1 transcribes a 95kDa protein that allow cadherins to anchor in intracellular cytoeskeleton (actin) by connection cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway (https://www.annualreviews.org/doi/full/10.1146/annurev.cellbio.20.010403.113126), related to embryonic development. Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [1]
| |||||||||||