Function
Hemoglobin is an oxygen-transport protein. Hemoglobin is an allosteric protein. It is a composed of two types of subunits designated α and β, with stoichiometry . The of hemoglobin sit roughly at the corners of a tetrahedron, facing each other across a at the center of the molecule. Each of the subunits prosthetic group. The give hemoglobin its red color.
Each individual molecule contains one atom. In the lungs, where oxygen is abundant, an binds to the ferrous iron atom of the heme molecule and is later released in tissues needing oxygen. The heme group binds oxygen while still attached to the . The spacefill view of the hemoglobin polypeptide subunit with an oxygenated heme group shows how the within the polypeptide.
is facilitated by a histidine nitrogen that binds to the iron. A second histidine is near the bound oxygen. The "arms" (propanoate groups) of the heme are hydrophilic and face the surface of the protein while the hydrophobic portions of the heme are buried among the hydrophobic amino acids of the protein.
Perhaps the most well-known disease caused by a mutation in the hemoglobin protein is sickle-cell anemia. It results from a mutation of the sixth residue in the β hemoglobin monomer from . This hemoglobin variant is termed 'hemoglobin S' (2hbs).
Hemoglobin subunit binding O2
For hemoglobin, its function as an oxygen-carrier in the blood is fundamentally linked to the equilibrium between the two main states of its quaternary structure, the unliganded "deoxy" or "T state" versus the liganded "oxy" or "R state". The unliganded (deoxy) form is called the "T" (for "tense") state because it contains extra stabilizing interactions between the subunits. In the high-affinity R-state conformation the interactions which oppose oxygen binding and stabilize the tetramer are somewhat weaker or "relaxed". In some organisms this difference is so pronounced that their Hb molecules dissociate into dimers in the oxygenated form. Structural changes that occur during this transition can illuminate how such changes result in important functional properties, such as cooperativity of oxygen binding and allosteric control by pH and anions. Hemoglobin is definitely not a pure two-state system, but the T to R transition provides the major, first-level explanation of its function.
The hemoglobin molecule (or "Hb") is a tetramer of two α and two β chains, of 141 and 146 residues in human. They are different but homologous, with a "globin fold" structure similar to myoglobin.
Here we see a single of hemoglobin, starting with an overview of the subunit. The 6 major and 2 short α-helices that make up the structure of a Hb subunit (the "globin fold") are , which is the traditional naming scheme. For example, the proximal histidine (the tightest protein Fe ligand) is often called , since it is residue 9 on helix F (it is residue 87 in the human α chain). The helices form an approximately-cylindrical bundle, with the heme and its central Fe atom bound in a .
the oxy (in pink) and deoxy (in deepskyblue) α1 heme groups were superimposed on each other, to give a local comparison at this site, a closeup around the heme O2-binding site. The heme is quite domed in the deepskyblue T-state (deoxy) form, with the 5-coordinate, high-spin Fe (orange ball) out of the plane. In the pink R-state form a CO molecule is bound at the right (C in green,O in red); the Fe, now 6-coordinate low-spin, has moved into the heme plane, which has flattenened. The proximal His (at left) connects the Fe to helices on the proximal side, making the Fe position sensitive to changes in the globin structure and vice versa. Remember that this scene shows a subunit in the all-unliganded versus the all-liganded states of Hb; when oxygen binds to just one subunit, then its internal structure undergoes some but not all of these changes, depending on conditions.
O2 binds in the same place as CO, with similar effects on the structure; however, for O2 the outer atom is angled rather than straight. The equilibrium between free and bound O2 is very rapid, with on and off rates that are sensitive to protein conformation. Both CO and NO dissociate from the Fe atom very slowly, so that these gases act as respiratory poisons. The α and β chains differ somewhat in their rates and relative affinities for O2 and other ligands, by virtue of heme-pocket differences, but the differences between affinities in the R vs T quaternary states are much larger.
Both α and β chains of Hb resemble myoglobin (the single-chain O2-binder in muscle), both in overall tertiary structure and in using an Fe atom centered in a heme group as the site where oxygen is reversibly bound. The heme is surrounded by a hydrophobic pocket, which is necessary in order for it to bind oxygen reversibly without undergoing oxidation or other undesirable reactions.
The heme binding pocket contains mostly , shown in grey. They actually surround the binding site so thoroughly that O2 cannot get in or out without parts of the protein moving out of the way a bit, so that its dynamic properties are essential to have any O2 binding at all; this restrictive process also increases the specificity of ligand binding.
The shift between R and T state requires subunit interactions and does not occur in myoglobin, or in isolated α or β chain monomers. These monomers bind O2 quite tightly, which would work well for loading O2 in the lungs but would not allow unloading it for delivery to the tissues. Therefore, the central critical feature of hemoglobin function is how it achieves, uses, and allosterically controls cooperativity between the 4 binding sites in the tetramer to tune O2 binding for satisfying physiological needs.
Linkage of the heme Fe through the proximal His results in tertiary-structure changes that can then transmit their effects to other subunits in the tetrameric assemblage. This allows O2 binding in one subunit to indirectly affect the affinitiy of other subunits. Briefly, inside the α chains the R/T equilibrium is reflected in changes in Fe spin state and position as it moves in or out of the heme plane; the proximal His changes distance and angle relative to the heme; the F helix shifts; Tyr 140 moves and its H-bond to backbone weakens; and both the C-terminus of the chain and Arg 141 move significantly at the interface. These movements are animated at this page. Changes at the subunit interface (coupled with changes at the Fe, as we have seen) alter the equilibrium between the deoxy and oxy quaternary structures, and conversely a change of quaternary structure alters the balance between the two states inside a given subunit. Each O2 that binds increases the likelihood of switching the tetramer into the oxy state, and once it switches, the O2 affinity at all sites increases because the local structure changes have either already occurred or are easier to make.
Click here to show the α1 subunit, but centered for the whole tetramer.
The Hb tetramer T -> R transition
The central cavity, is wider in the deoxy state, forming phosphate sites; quaternary structure change as rigid rotations of α-β dimers; α1-β2 contact overview; "ratchet" vs "hinge" at the a1b2 interface; α1-α2 salt bridges; charged groups at the C-terminus of β2 which stabilize the deoxy form; and finally a summary overview. (from PDB files bio3HHB and bio1HCO)
Look down one of the approximate 2-fold axes, with α subunits at the top and β subunits at the bottom. Notice that the hemes are quite far apart, so that their interactions must be mediated by the protein.
For a view down the exact crystallographic 2-fold axis from the β1- β2 end, click here: The yellowtint crosses are phosphate sites present in deoxy but not oxy Hb. In oxy Hb, the β subunits move closer together, squeezing out phosphates (such as 2,3 DPG), and allowing the N- and C-termini to interact. DPG and other phosphates bind much more strongly to the deoxy quaternary structure; therefore they necessarily push the equilibrium toward deoxy Hb, and because of that they decrease O2 affinity. Such regulatory phosphate molecules are useful in the blood, because their concentrations can be controlled to shift the Hb O2-binding curve so that it is working across the steepest and most efficient part under conditions in the lungs and tissues. For instance, at high altitude the body makes more DPG, to unload O2 more effectively in the muscles.
Like the PFK, to the first approximation the Hb molecule consists of two "dimers" (α1-β1 and α2-β2), which rotate relative to each other as rigid bodies in the R-T transition. The α1-β1 unit undergoes relatively little internal rearrangement, but its overall rotation with respect to the α2-β2 unit is considerable. The net rotation of the two dimers alters their interactions with one another, most notably at the allosteric effector site between β1 and β2 (PO4 binding) and at the important α1-β2 interface, where mutations have the largest effect on Hb allosteric properties. Although the symmetry is not exact, similar parts of the subunits contact each other: the C helix, and the "FG corner" between helices F and G.
Have a look at a closeup that emphasizes the ratchet contact between the C helix of α1 and the FG corner of β2; His 97 of the β2 FG corner makes a large jump against Thr 38 and Thr 41 of the α1 C helix. In a closeup of the hinge contact, the motions are mainly rotations without much shift, between the α1 FG corner and the β2 C helix. Labels help identify these parts. Since this is a complex motion orchestrated between the fit of two quite different sets of contacts in the two states, this interface is critical to making Hb allostery work, and mutations of residues in this interface have been found to be especially likely to influence cooperativity and allostery.
There are salt links between α1 and α2, which stabilize the deoxy form. Here’s an overview down the exact 2-fold axis between the subunits, showing that there are two equivalent sets of interactions, on either side of the twofold.
Salt links at the C-terminus of β2 stabilize the deoxy T form and make a large contribution to the pH dependence of Hb oxygen binding, known as the Bohr Effect. In the making and breaking of these interactions, His β 146 moves a great deal, disrupting the salt link (charged H-bond) to Asp β 94 that is formed in the T state. Since His titrates near physiological pH, this interaction is quite pH sensitive. At low pH, when more protons are present, the His ring N is more likely to be protonated and positive; this strengthens its H-bond with Asp 94, thus favoring the T state and decreasing O2 affinity. The pH effect, or Bohr Effect, can be considered as allosteric regulation by the binding of protons. It is important biologically, because it promotes oxygen unloading in the tissues where proton concentrations are elevated, for instance by the production of lactic acid in muscle.
Truncated hemoglobins
, also known as 2/2 hemoglobins, can be further classified into three different groups (I, II, and III). Genomic sequences of bacteria, cyanobacteria, and plants indicate that trHbs are rather common. Group I, Group II, and Group III trHbs have distinct phylogenetic trees and show different ligand-binding properties. The Group I trHb of the ciliated protozoan Tetrahymena pyriformis (Tp trHb) was first discovered by Keilin and Ryley in 1953.
It is known that trHbs exist in ciliates of the Tetrahymena group, but trHb structure and function remain poorly understood. To investigate trHb function with respect to stability of bound oxygen and protein structure, we measured the oxygen binding kinetics of Tetrahymena pyriformis trHb, and determined the crystal structure of the protein.
The three-dimensional structure of an was determined at 1.73 Å resolution (3aq9). . Tyr25 donated a hydrogen bond to the terminal oxygen atom, whereas Gln46 hydrogen-bonded to the proximal oxygen atom. Furthermore, .
The O2 association and dissociation rate constants of T. pyriformis trHb were 5.5 μM-1 s-1, and 0.18 s-1, respectively. The oxygen affinity was determined to be 33 nM. The autooxidation rate constant was 3.8 x 10-3 h-1. These values are similar to those of .
Mutations:
- Mutation at Tyr25: and .
- Mutation at Gln46:
- Mutation at increased the O2 dissociation and autooxidation rate constants, and partly disrupted the hydrogen-bonding network.
An , in a crystal state, with nitric oxide. This suggests that Tp trHb functions in nitric oxide detoxification.
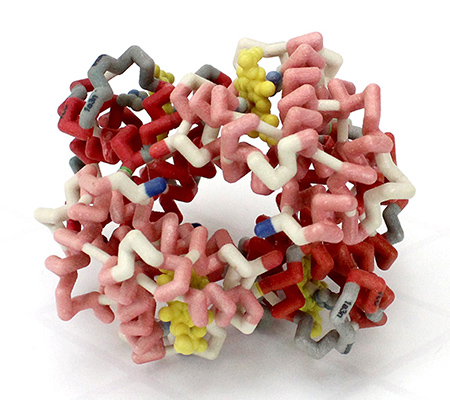
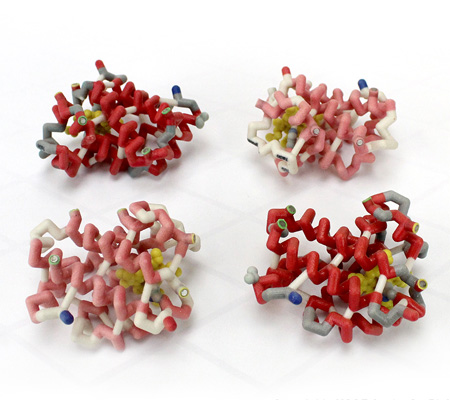
3D Printed Physical Model of Hemoglobin at The MSOE Center for BioMolecular Modeling
Shown below is a 3D printed physical model of Hemoglobin, based on the structure 1a3n.pdb. The two alpha-globin chains are colored light red, the two beta globin chains are colored dark red, and the four heme groups are colored yellow. It has been designed with precisely embedded magnets that allow the four chains to pull apart into individual pieces.
Additional Resources
Hemoglobin 3D structures
See Hemoglobin 3D structures.



