User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
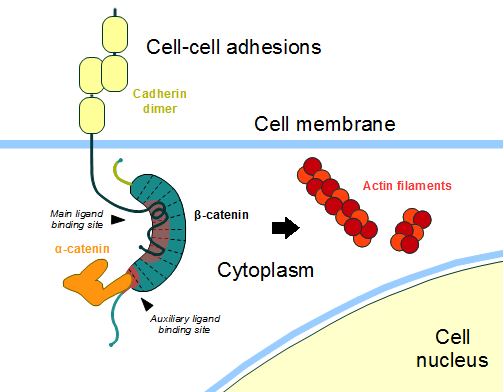
In the absence of Wnt stimulus, the ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin (but alpha-catenin, p120-catenin too) by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted, in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT). <ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> | In the absence of Wnt stimulus, the ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin (but alpha-catenin, p120-catenin too) by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted, in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT). <ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> | ||
| - | The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X]) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | + | The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X, from ''Mus musculus'']) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> |
[[Image:Beta-catenin-moonlighting.png]] | [[Image:Beta-catenin-moonlighting.png]] | ||
| Line 34: | Line 34: | ||
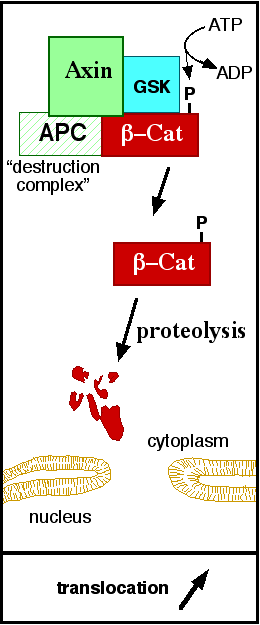
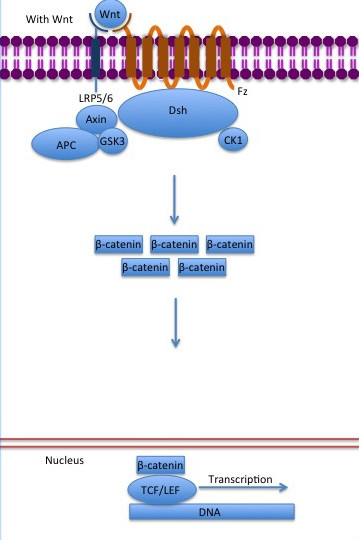
The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | ||
| - | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT (orange), which interacts with the central ARM repeat of ß-catenin (green).</scene> ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | + | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT (orange), which interacts with the central ARM repeat of ß-catenin (green).</scene> ([http://www.rcsb.org/structure/1M1E 1M1E, from "Mus musculus" and "Homo sapiens"]) <ref name="valenta2012" /> |
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
Revision as of 12:51, 9 July 2020
ß-catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||