User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
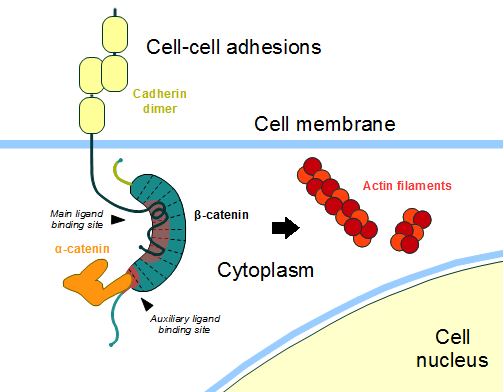
In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted: in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT).<ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> | In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 1). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted: in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT).<ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> | ||
| - | The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') (1I7X). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | + | The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') ([https://www.rcsb.org/structure/1i7x 1I7X]). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> |
[[Image:Beta-catenin-moonlighting.png]] | [[Image:Beta-catenin-moonlighting.png]] | ||
Revision as of 19:35, 9 July 2020
ß-catenin
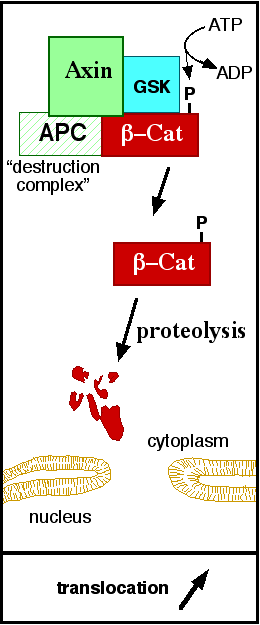
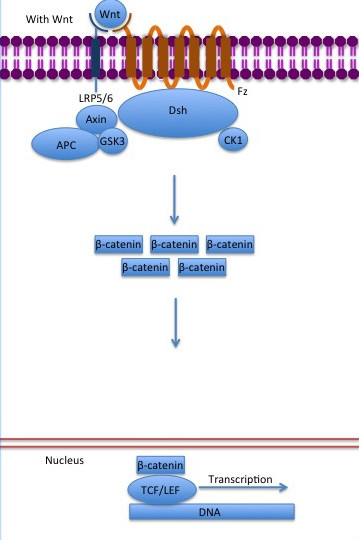
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||