User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | ||
| + | |||
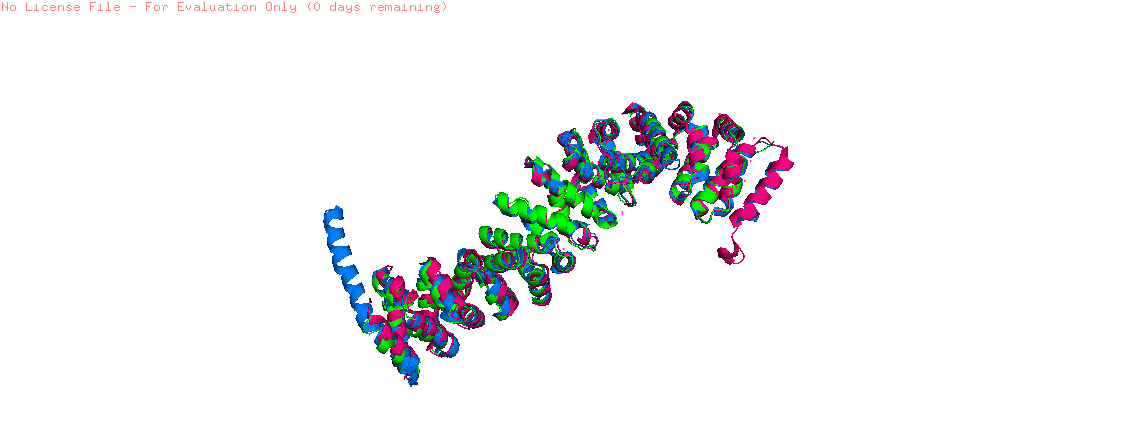
| + | Finally, the ''Danio rerio'' (full length structure, blue), ''Mus musculus'' (armadillo repeat region, green) and ''Homo sapiens'' (full length structure, pink) beta-catenin alignment (Figure 1) shows that the protein structure is quite similar in these organisms. The three structures have 12 armadillo repeat group and the superposition indicates that the helix C in zebrafish and human beta-catenin conformation and orientation are essentially the same in both crystal structures. This great similarity between these proteins demonstrates that beta-catenin is evolutionary conserved and so are the pathways that it takes part. | ||
| + | |||
| + | [[Image:2z6g 2bct 2z6h white.png]] | ||
| + | '''Figure 1''': Superposition of a full length zebrafish (shown in blue), full length human (pink) and armadillo repeat region mouse (green) beta-catenin. | ||
==Cell Adhesion== | ==Cell Adhesion== | ||
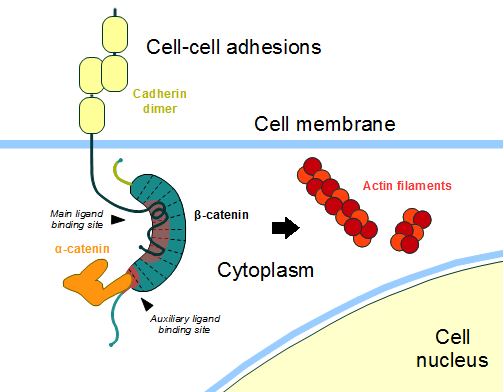
| - | In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure | + | In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 2). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted: in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT).<ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> |
The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') ([https://www.rcsb.org/structure/1i7x 1I7X]). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') ([https://www.rcsb.org/structure/1i7x 1I7X]). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | ||
[[Image:Beta-catenin-moonlighting.png]] | [[Image:Beta-catenin-moonlighting.png]] | ||
| - | '''Figure | + | '''Figure 2''': Cadherin-based cell adhesion. Alpha-catenin/ß-catenin forms a heterodimer that can connects to E-cadherin promoting the adherens junctions. As a homodimer, alpha-catenin interacts with actin. Adapted from: Bubus12/CC BY (https://creativecommons.org/licenses/by/3.0). https://commons.wikimedia.org/wiki/File:Beta-catenin-moonlighting.png |
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
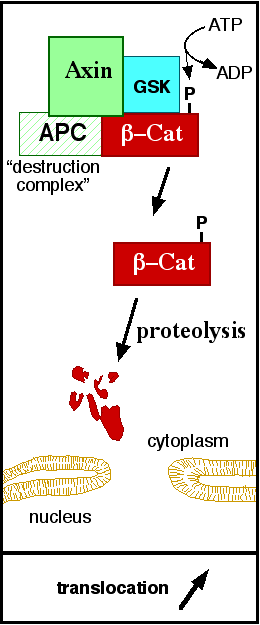
| - | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure | + | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 3), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. <ref name="valenta2012" /> Furthermore, the relation Wnt/ß-catenin pathway usually are reported by 'canonical' and 'non-canonical', whose meaning remotes to the components of the cascate. The first leads to accumulation and stabilization of cytosolic (unphosphorylated) ß-catenin and the second promotes the increase in intracellular calcium or mediate cell polarity, but both are established in embryonic development of normal tissue and organs. <ref name=Arend ''et al''2013> Arend ''et al,''2013. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecologic Oncology. Volume 131, Issue 3, December 2013, Pages 772-779.</ref> <ref name=Takayama ''et al''1996>Takayama ''et al,'' 1996. ß-Catenin Expression in Human Cancers. American journal of Pathology, Vol. 148, No. 1, January. </ref> |
[[Image:Axindestructioncomplex.png]] | [[Image:Axindestructioncomplex.png]] | ||
| - | '''Figure | + | '''Figure 3''': A simplified diagram of the ß-catenin destruction complex. The destruction complex proteins promote the ß-catenin proteolysis in cytoplasm. Source: JWSchmidt at the English language Wikipedia/CC BY-SA (https://creativecommons.org/licenses/by/3.0). https://commons.wikimedia.org/wiki/File:Axindestructioncomplex.png |
==DNA binding and transcription== | ==DNA binding and transcription== | ||
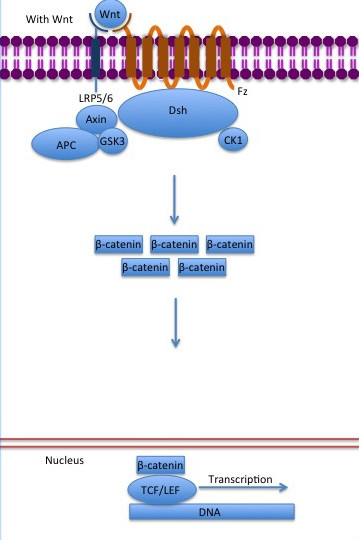
| - | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure | + | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 4) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> |
TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | ||
| Line 37: | Line 42: | ||
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
| - | '''Figure | + | '''Figure 4''': The canonical Wnt pathway when Wnt is present. The inhibition of the destruction complex allows ß-catenin translocation from cytoplasm to nucleus. Source: Gpruett2/CC BY-SA (https://creativecommons.org/licenses/by/3.0). https://commons.wikimedia.org/wiki/File:Canonical_Wnt_pathway_with_Wnt..jpg |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 20:23, 5 August 2020
ß-catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||