We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jeremiah C Hagler/Protein Visualization Lab COVID
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
<br> | <br> | ||
[[Image:Intramolecular forces in tertiary structures.png]] | [[Image:Intramolecular forces in tertiary structures.png]] | ||
| - | Figure 3: The various intramolecular interactions that help determine | + | Figure 3: The various intramolecular interactions that help determine tertiary structure |
Proteins may contain only alpha helices, only beta sheets, or a combination of the two. The same holds true for the bonds giving a protein its tertiary structure - all, some or none may be present. These different folding patterns existing in different proteins are what give the proteins their distinctive shapes and sizes. A protein that is 300 amino acids long will be 100 nm as an extended chain. If the protein is an alpha helix, it will be 45 nm long; a beta sheet will be 7 x 7 x 0.8 nm; and a small globular form will form a sphere only 4.5 nm in diameter! | Proteins may contain only alpha helices, only beta sheets, or a combination of the two. The same holds true for the bonds giving a protein its tertiary structure - all, some or none may be present. These different folding patterns existing in different proteins are what give the proteins their distinctive shapes and sizes. A protein that is 300 amino acids long will be 100 nm as an extended chain. If the protein is an alpha helix, it will be 45 nm long; a beta sheet will be 7 x 7 x 0.8 nm; and a small globular form will form a sphere only 4.5 nm in diameter! | ||
| - | Finally, multiple proteins might interact to form a quaternary structure ( | + | Finally, multiple proteins might interact to form a quaternary structure (4°), sometimes known as a protein complex. Each protein in a quaternary structure is called a subunit, and in some cases multiple subunits of the same protein interact to form a quaternary structure (as in the SARS-CoV-2 spike protein you will learn more about below). On the other hands, some quaternary structures contain multiple types of proteins that interact. Most quaternary structures are held together by weak intermolecular bonds (ionic, H-bonds, hydrophobic, van der waals, etc) and occasionally by strong covalent bonds (such as disulfide bonds). |
Domains: | Domains: | ||
Parts of the secondary and tertiary structures of a protein are usually arranged to form domains, functional units associated with a particular structure. For example, a pair of alpha helices situated side by side might form a binding site, or a particular folding pattern might form the active site of an enzyme, where it binds to its substrate, or the site at which it binds to a coenzyme such as NAD+. The structure of the domain (though not necessarily the exact amino acid sequence) is frequently preserved in different proteins from the same organism that have a similar function (to bind to a cellular receptor, for example, in the SARS-CoV-2 Spike Protein, or to move phosphate groups, for instance). Domains are also conserved in proteins from different species that have the same function (such as hemoglobins for oxygen transport or cytochromes in the electron transfer system of mitochondria). Variations in the amino acid sequences in similar domains (or in the nucleotide sequences or genes that code for the proteins) give important clues about evolutionary relationships between organisms. Individual domains are sometimes found (but not always, a fact that makes this a very controversial topic) contained within single exons of eukaryotic genes (exons and introns are concepts you will learn more about later in the course, when we discuss eukaryotic gene structure). In other words, a single exon might represent all of the protein coding sequence required to generate a functional domain within the context of the whole protein structure. This finding has implications for the evolution of eukaryotic genes, since it implies that new proteins can be generated by simply duplicating preexisting protein domain encoding exons and recombining them into new combinations (a process known as exon-shuffling). Thus, a vast variety of proteins with new functions can be generated from preexisting genes, allowing great evolutionary flexibility. Looking at the genes of many eukaryotic organisms shows that this is exactly what appears to happen. | Parts of the secondary and tertiary structures of a protein are usually arranged to form domains, functional units associated with a particular structure. For example, a pair of alpha helices situated side by side might form a binding site, or a particular folding pattern might form the active site of an enzyme, where it binds to its substrate, or the site at which it binds to a coenzyme such as NAD+. The structure of the domain (though not necessarily the exact amino acid sequence) is frequently preserved in different proteins from the same organism that have a similar function (to bind to a cellular receptor, for example, in the SARS-CoV-2 Spike Protein, or to move phosphate groups, for instance). Domains are also conserved in proteins from different species that have the same function (such as hemoglobins for oxygen transport or cytochromes in the electron transfer system of mitochondria). Variations in the amino acid sequences in similar domains (or in the nucleotide sequences or genes that code for the proteins) give important clues about evolutionary relationships between organisms. Individual domains are sometimes found (but not always, a fact that makes this a very controversial topic) contained within single exons of eukaryotic genes (exons and introns are concepts you will learn more about later in the course, when we discuss eukaryotic gene structure). In other words, a single exon might represent all of the protein coding sequence required to generate a functional domain within the context of the whole protein structure. This finding has implications for the evolution of eukaryotic genes, since it implies that new proteins can be generated by simply duplicating preexisting protein domain encoding exons and recombining them into new combinations (a process known as exon-shuffling). Thus, a vast variety of proteins with new functions can be generated from preexisting genes, allowing great evolutionary flexibility. Looking at the genes of many eukaryotic organisms shows that this is exactly what appears to happen. | ||
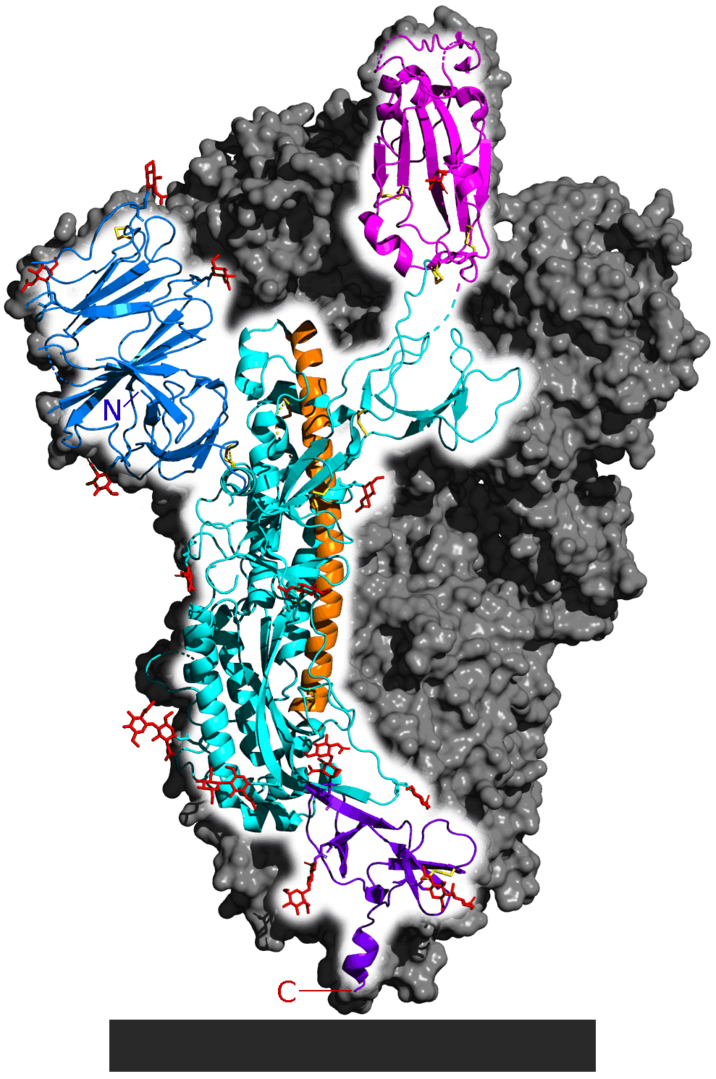
| - | + | A good example of all of these principles can be found in the SARS-CoV-2 Spike protein (see figure 4). This is the protein the SARS-CoV-2 virus relies on to gain entrance into its target......that target being any cell that contains the Angiotensin-converting enzyme 2 (ACE2) protein on its surface. The Spike protein is made up of three identical strands of protein (each called a subunit) arranged into a quaternary structure. Each is synthesized as an individual protein and then later complexed into the complex secondary, tertiary and quaternary structure you see below. Each individual subunit of the spike protein is divided into four major domains, S1 (or receptor-binding domain), S2 (membrane-fusion domain), TM (trans-membrane anchor) and IC (intracellular-tail). | |
| - | Figure 4: | + | Figure 4: SARS-CoV-2 Spike Protein representations. |
<br> | <br> | ||
| - | a. [[Image: | + | a. [[Image:PPC_Motif_in_SARS-CoV-2_Spike_Protein_Cropped_to_Spike_Schematic_Only_B.jpg|300px|]] |
<br> | <br> | ||
| - | + | Schematic drawing of the three-dimensional (3D) structure of SARS-CoV-2 coronavirus spike. S1, receptor-binding domain; S2, membrane fusion domain; TM, transmembrane anchor; IC, intracellular tail. | |
<br> | <br> | ||
| - | + | c. [[Image:716px-6VSB_spike_protein_SARS-CoV-2_monomer_in_homotrimer.png|6VSB spike protein SARS-CoV-2 monomer in homotrimer]] | |
| - | + | <scene name='71/713432/Antibody/1'>3-Dimensional structure of IgG antibody</scene>This is the same structure represented in a ribbon diagram that shows secondary structure. The flat ribbons show beta-sheets while the cylindrical barrels represent alpha-helices. The two heavy chains are in blue, the two light chains in green. The variable domains are the section of the structure where the light chains interact with the heavy chain. This is where antibodies bind to antigen. The constant domain consists of the region where the two heavy chains interact with each other. The interaction of secondary structure to form tertiary structure, and the interaction of these structures to form quaternary structure is apparent. The final step of protein folding results in quarternary structure (or 4° structure). This step is only taken in proteins that are made of multiple subunits; meaning that strands of proteins - coded for on separate mRNAs and synthesized independently - come together to form a single functional molecule. Many proteins have multiple subunits; for example, immunoglobulins are made up of four subunits (figure 4). | |
| - | + | ||
| - | + | ||
| - | + | ||
== Determining the 3-Dimensional Structure of a Protein == | == Determining the 3-Dimensional Structure of a Protein == | ||
Revision as of 20:44, 17 September 2020
Introduction to Computer-Aided Protein Visualization Lab
| |||||||||||