This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox GGC5
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=='''Titin'''== | =='''Titin'''== | ||
<StructureSection load='1TIT' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1TIT' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | Titin,one of the largest human protein, in its longest isoform, has a molecular weight exceeding 3 MDa and is over 1.5 μm in length. Titin typically contains immunoglobulin (Ig) domains which are typically 110 amino acids in length, contain an internal disulfide bond and two layers of β-pleated sheets.<ref>PMID:31856237</ref> On the cellular level, titin is typically located within the nucleus of the cell; however, it can also be located within the cytoplasm. <ref>DOI 10.1002/ijch.201300024</ref> | + | Titin, one of the largest human protein, in its longest isoform, has a molecular weight exceeding 3 MDa and is over 1.5 μm in length. Titin typically contains immunoglobulin (Ig) domains which are typically 110 amino acids in length, contain an internal disulfide bond and two layers of β-pleated sheets.<ref>PMID:31856237</ref> On the cellular level, titin is typically located within the nucleus of the cell; however, it can also be located within the cytoplasm. <ref>DOI 10.1002/ijch.201300024</ref> |
== '''Function''' == | == '''Function''' == | ||
| Line 38: | Line 38: | ||
== '''Relevance''' == | == '''Relevance''' == | ||
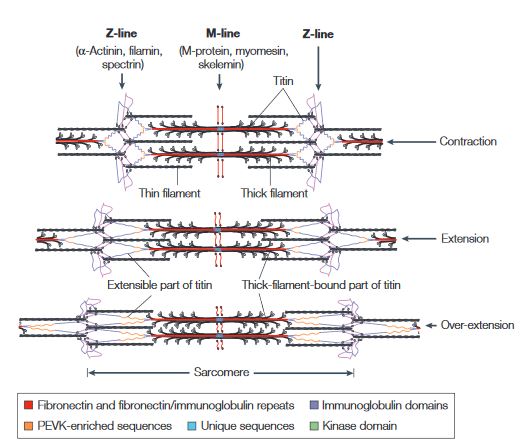
| + | Titin is a flexible filament containing a beaded substructure indicating the presence of multiple domains within the molecule. These multiple domains include: the immunoglobulin domain, the Fibronectin type-II domain, the PEVK (proline-glutamate-valine-lysine-enriched unique sequence region, the unique sequences and the kinase domain. <ref name="figure" /> | ||
| + | |||
[[Image:Titin.JPG]] | [[Image:Titin.JPG]] | ||
'''This figure illustrates the ability of titin to coil and extend during muscle contraction and extension.'''<ref name="figure">PMID:10895161</ref> | '''This figure illustrates the ability of titin to coil and extend during muscle contraction and extension.'''<ref name="figure">PMID:10895161</ref> | ||
| - | + | ||
| - | + | When the muscle contracts, the sarcomere length decreases resulting in the coiling of the I-band part of the titin. As the muscle extends, the sarcomere length increases resulting in the extension of titin. In the event of over-extension of the muscles there is unravelling of the titin peptide, starting in the least mechanically stable PEVK domain. <ref name="figure" /> | |
| + | |||
== '''Structural highlights''' == | == '''Structural highlights''' == | ||
Revision as of 02:25, 13 November 2020
Titin
| |||||||||||
References
- ↑ Chatziefthimiou SD, Hornburg P, Sauer F, Mueller S, Ugurlar D, Xu ER, Wilmanns M. Structural diversity in the atomic resolution 3D fingerprint of the titin M-band segment. PLoS One. 2019 Dec 19;14(12):e0226693. doi: 10.1371/journal.pone.0226693., eCollection 2019. PMID:31856237 doi:http://dx.doi.org/10.1371/journal.pone.0226693
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Tskhovrebova L, Trinick J. Giant proteins: sensing tension with titin kinase. Curr Biol. 2008 Dec 23;18(24):R1141-2. doi: 10.1016/j.cub.2008.10.035. PMID:19108772 doi:http://dx.doi.org/10.1016/j.cub.2008.10.035
- ↑ 4.0 4.1 Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998 Oct 29;395(6705):863-9. PMID:9804419 doi:10.1038/27603
- ↑ Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005 Jun 10;308(5728):1599-603. Epub 2005 Mar 31. PMID:15802564 doi:1110463
- ↑ 6.0 6.1 Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem Biophys Res Commun. 1999 Aug 27;262(2):411-7. PMID:10462489 doi:10.1006/bbrc.1999.1221
- ↑ Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T, Takahashi M, Hohda S, Ueda K, Nouchi T, Hiroe M, Marumo F, Imaizumi T, Yasunami M, Kimura A. Titin mutations as the molecular basis for dilated cardiomyopathy. Biochem Biophys Res Commun. 2002 Feb 22;291(2):385-93. PMID:11846417 doi:10.1006/bbrc.2002.6448
- ↑ 8.0 8.1 Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002 Sep;71(3):492-500. Epub 2002 Jul 26. PMID:12145747 doi:S0002-9297(07)60330-9
- ↑ Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007 Apr;61(4):340-51. PMID:17444505 doi:10.1002/ana.21089
- ↑ 10.0 10.1 10.2 10.3 Hu LF, Chen F, Altiok E, Winberg G, Klein G, Ernberg I. Cell phenotype-dependent splicing reflecting differential promoter usage for EBNA transcripts in EBV-carrying cells. Gan To Kagaku Ryoho. 2000 May;27 Suppl 2:248-60. PMID:10895161
- ↑ Improta S, Politou AS, Pastore A. Immunoglobulin-like modules from titin I-band: extensible components of muscle elasticity. Structure. 1996 Mar 15;4(3):323-37. PMID:8805538