We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Spike glycoprotein== | ==Spike glycoprotein== | ||
<StructureSection load='6VSB' size='340' side='right' caption='3D representation of the Spike glycoprotein' scene=''> | <StructureSection load='6VSB' size='340' side='right' caption='3D representation of the Spike glycoprotein' scene=''> | ||
| - | + | 3D structure representation of the Spike glycoprotein related to the SARS-CoV-2 <ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref>. | |
==Introduction== | ==Introduction== | ||
| Line 16: | Line 16: | ||
==Activation of S-protein== | ==Activation of S-protein== | ||
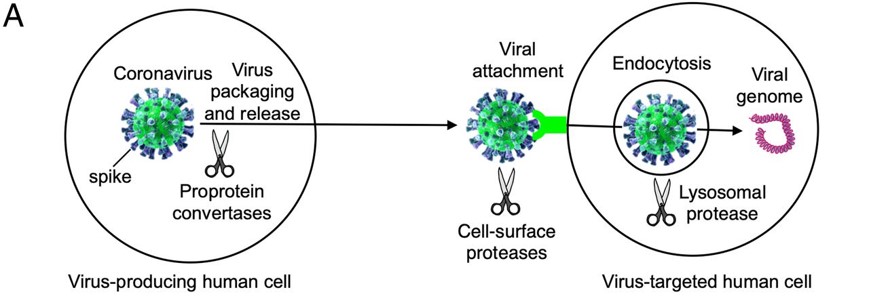
| - | + | Before the Spike protein can be activated , it has to be cleaved by the protease Furin protein ALA 668 <scene name='75/752266/Ala_668/1'>Furin Cleavage site</scene> . This 2D image below shows the schematic cleavage of the S-protein before and after it enters the host cell | |
[[Image:Cleavage.jpg]] <ref>Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., & Li, F. (2020, May 26). Cell entry mechanisms of SARS-CoV-2. Retrieved November 14, 2020, from https://www.pnas.org/content/117/21/11727</ref> | [[Image:Cleavage.jpg]] <ref>Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., & Li, F. (2020, May 26). Cell entry mechanisms of SARS-CoV-2. Retrieved November 14, 2020, from https://www.pnas.org/content/117/21/11727</ref> | ||
| Line 23: | Line 23: | ||
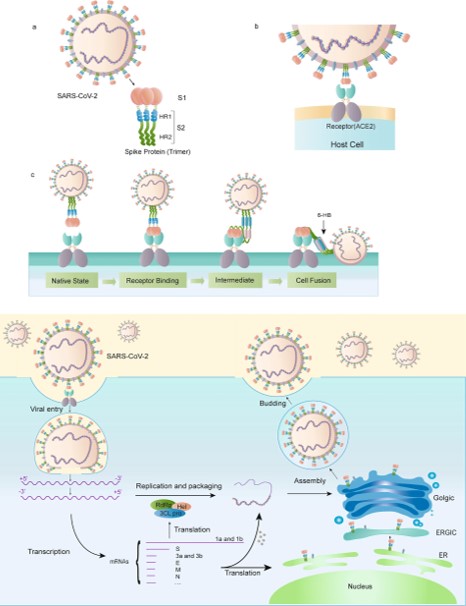
LYS 187 <scene name='75/752266/Lys_187/1'>The Receptor Binding Domain (RBD)</scene> | LYS 187 <scene name='75/752266/Lys_187/1'>The Receptor Binding Domain (RBD)</scene> | ||
| - | [[Image:Picture3.jpg]]<ref> | + | [[Image:Picture3.jpg]]<ref>doi:10.1038/s41401-020-0485-4</ref> |
==Mutation== | ==Mutation== | ||
| - | + | The most studied mutation site of the S-protein is at residue 614 which encodes for the amino acid Aspartic acid (D) D614 <scene name='75/752266/Asp_614/1'>The Mutation site D614 </scene> and is normally changed to Glycine (G). And this form of mutation causes the enhancement of the viral transmission <ref>doi.org/10.1016/j.ijid.2020.10.033</ref>. | |
| Line 34: | Line 34: | ||
== Structural highlights == | == Structural highlights == | ||
| - | These are the structural 3D representations of the s-protein showing the two subunits , the binding regions to its receptor human ACE2 , Mutation site , RBD site and cleavage site respectively NTD-CT<scene name='75/752266/Ntd_-_ct/1'>S1 subunit is in the downstream of NTD and S2 subunit is in the upsteam of CT</scene> .Val367<scene name='75/752266/Val367/1'>Binding region val367</scene> . D614 <scene name='75/752266/Asp_614/1'>The Mutation site D614 </scene>. LYS 187 <scene name='75/752266/Lys_187/1'>The Receptor Binding Domain (RBD)</scene>.ALA 668 <scene name='75/752266/Ala_668/1'>Furin Cleavage site</scene> | + | These are the structural 3D representations of the s-protein showing the two subunits , the binding regions to its receptor human ACE2 , Mutation site , RBD site and cleavage site respectively. NTD-CT<scene name='75/752266/Ntd_-_ct/1'>S1 subunit is in the downstream of NTD and S2 subunit is in the upsteam of CT</scene> .Val367<scene name='75/752266/Val367/1'>Binding region val367</scene> . D614 <scene name='75/752266/Asp_614/1'>The Mutation site D614 </scene>. LYS 187 <scene name='75/752266/Lys_187/1'>The Receptor Binding Domain (RBD)</scene>.ALA 668 <scene name='75/752266/Ala_668/1'>Furin Cleavage site</scene> |
</StructureSection> | </StructureSection> | ||
Revision as of 18:05, 16 November 2020
Spike glycoprotein
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Huang, Y., Yang, C., Xu, X., Xu, W., & Liu, S. (2020). Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica, 41(9), 1141-1149. doi:10.1038/s41401-020-0485-4
- ↑ Mohammad, A., Alshawaf, E., Marafie, S. K., Abu-Farha, M., Abubaker, J., & Al-Mulla, F. (2020). Higher binding affinity of Furin to SARS-CoV-2 spike (S) protein D614G could be associated with higher SARS-CoV-2 infectivity. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, S1201-9712(20)32237-2. Advance online publication. https://doi.org/10.1016/j.ijid.2020.10.033
- ↑ UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. (2020, October 07). Spike glycoprotein. Retrieved November 13, 2020, from https://www.uniprot.org/uniprot/P59594
- ↑ Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., & Li, F. (2020, May 26). Cell entry mechanisms of SARS-CoV-2. Retrieved November 14, 2020, from https://www.pnas.org/content/117/21/11727
- ↑ Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020 Sep;41(9):1141-1149. doi: 10.1038/s41401-020-0485-4., Epub 2020 Aug 3. PMID:32747721 doi:http://dx.doi.org/10.1038/s41401-020-0485-4
- ↑ doi.org/10.1016/j.ijid.2020.10.033