Tetrahydroprotoberberine N-methyltransferase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
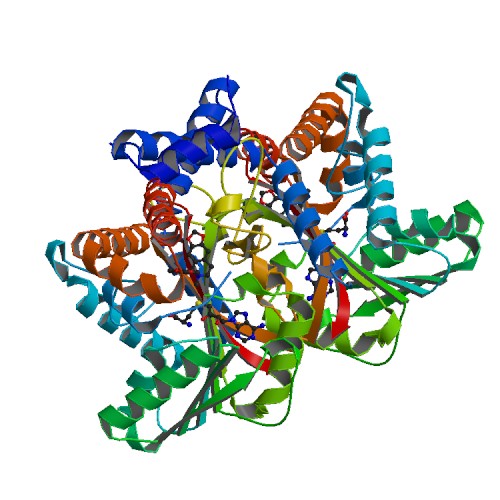
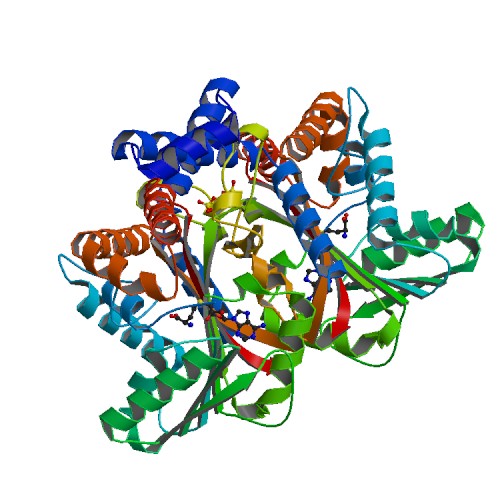
| - | <StructureSection load='6P3N' size='340' side='right' caption=' | + | <StructureSection load='6P3N' size='340' side='right' caption='Tetrahydroprotoberberine complex with SAM (PDB code [[6p3n]])' scene=''> |
= Introduction = | = Introduction = | ||
'''Tetrahydroprotoberberine N-methyltransferase''' is a protein whose dimer interface include 6 salt bridges and 8 hydrogens bonds. It is expressed in E. ''coli'' and crystallized at a pH of 7.0. The crystals were grown in the presence of SAH,SAM, and SAH+SMS. Below are the two different substrates that were in the presence of the crystallized protein. The substrate SAM is shown to the right. | '''Tetrahydroprotoberberine N-methyltransferase''' is a protein whose dimer interface include 6 salt bridges and 8 hydrogens bonds. It is expressed in E. ''coli'' and crystallized at a pH of 7.0. The crystals were grown in the presence of SAH,SAM, and SAH+SMS. Below are the two different substrates that were in the presence of the crystallized protein. The substrate SAM is shown to the right. | ||
Revision as of 07:57, 25 November 2020
| |||||||||||
3D structures of tetrahydroprotoberberine N-methyltransferase
Updated on 25-November-2020
6p3m – GfTHPBM + SAH – Glaucum flavum
6p3n – GfTHPBM + SAM
6p3n – GfTHPBM + SAH + methylstylopine
References
- ↑ Takao, N., Kamigauchi, M., and Okada, M. (1983) Biosynthesis of benzo-[c]phenanthridine alkaloids sanguinarine, chelirubine and macarpine.Helv. Chim. Acta 66, 473–484 CrossRef
- ↑ Bennett, M. R., Thompson, M. L., Shepherd, S. A., Dunstan, M. S., Herbert, A. J., Smith, D. R. M., Cronin, V. A., Menon, B. R. K., Levy, C., and Micklefield, J. (2018) Structure and biocatalytic scope of coclaurine Nmethyltransferase.Angew. Chem. Int. Ed. Engl. 57, 10600–10604CrossRef Medline