We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1628
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
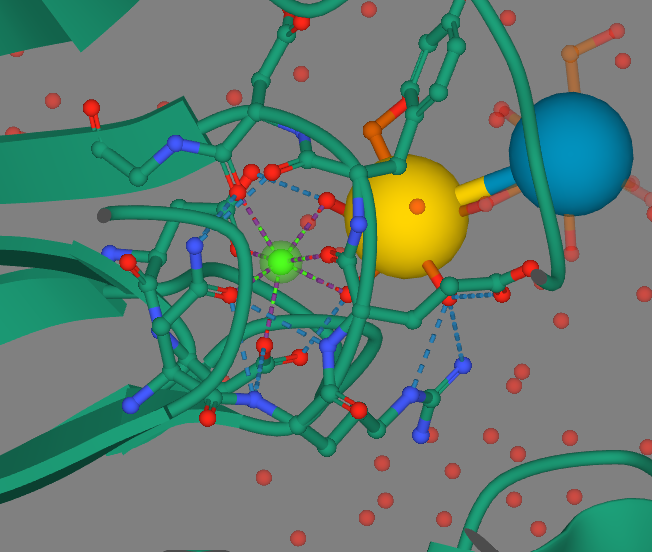
The majority of the <scene name='86/861610/Secondarystructure/1'>protein's secondary structure</scene> is comprised of antiparallel beta sheets with loops connecting them. Two loops help to form a calcium binding pocket which helps to bind the calcium ion shown and three other loops form the outer pocket. These loops are highly variable in their composition and are contributed with recognizing certain sugars that the inner binding site prefer to bind to. | The majority of the <scene name='86/861610/Secondarystructure/1'>protein's secondary structure</scene> is comprised of antiparallel beta sheets with loops connecting them. Two loops help to form a calcium binding pocket which helps to bind the calcium ion shown and three other loops form the outer pocket. These loops are highly variable in their composition and are contributed with recognizing certain sugars that the inner binding site prefer to bind to. | ||
| - | The tertiary structure allows the loops created by the antiparallel beta sheets <scene name='86/861610/Tertiarystructure/1'>to come close enough to interact with one another</scene>. Between two of the variable loops is a disulfide bond created by two cystine amino acids which is the only conserved area within the loops. The disulfide bond helps to maintain structure of the outer pocket and is consistent between a variety of the epithelial adhesins. | + | The tertiary structure allows the loops created by the antiparallel beta sheets <scene name='86/861610/Tertiarystructure/1'>to come close enough to interact with one another</scene>. These loops help to stabilize the calcium ion shown in blue as well as form the outer pocket. Between two of the variable loops is a disulfide bond created by two cystine amino acids which is the only conserved area within the loops. The disulfide bond helps to maintain structure of the outer pocket and is consistent between a variety of the epithelial adhesins. |
The protein itself has <scene name='86/861610/Spacefill/2'>a small indent near the active site</scene> where the calcium ion sits just sticking out to interact with sugar ligands. The cleft into the protein fits a very specific size of sugar, which follows along with the current research that shows only a few different pyranose sugars can interact with the adhesins<ref name="journal" />. | The protein itself has <scene name='86/861610/Spacefill/2'>a small indent near the active site</scene> where the calcium ion sits just sticking out to interact with sugar ligands. The cleft into the protein fits a very specific size of sugar, which follows along with the current research that shows only a few different pyranose sugars can interact with the adhesins<ref name="journal" />. | ||
Current revision
| This Sandbox is Reserved from 09/18/2020 through 03/20/2021 for use in CHEM 351 Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, IA. This reservation includes Sandbox Reserved 1628 through Sandbox Reserved 1642. |
To get started:
More help: Help:Editing |
Epithelial Adhesin 1A
| |||||||||||
References
- ↑ Soto GE, Hultgren SJ. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999 Feb;181(4):1059-71. doi: 10.1128/JB.181.4.1059-1071.1999. PMID:9973330 doi:http://dx.doi.org/10.1128/JB.181.4.1059-1071.1999
- ↑ 2.0 2.1 Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999 Jan;12(1):80-96. PMID:9880475
- ↑ 3.0 3.1 Hoffmann D, Diderrich R, Reithofer V, Friederichs S, Kock M, Essen LO, Mosch HU. Functional reprogramming of Candida glabrata epithelial adhesins: the role of conserved and variable structural motifs in ligand binding. J Biol Chem. 2020 Jul 15. pii: RA120.013968. doi: 10.1074/jbc.RA120.013968. PMID:32669365 doi:http://dx.doi.org/10.1074/jbc.RA120.013968