Sandbox Reserved 1661
From Proteopedia
| Line 22: | Line 22: | ||
== HGH receptors and interactions == | == HGH receptors and interactions == | ||
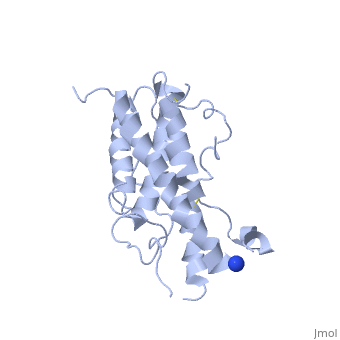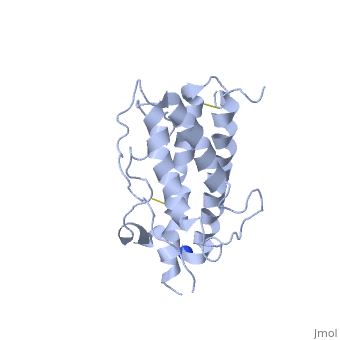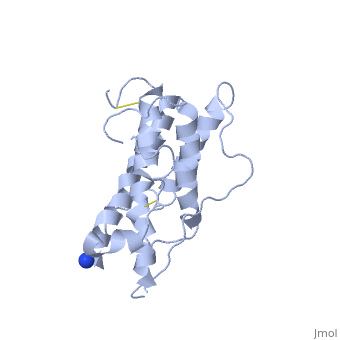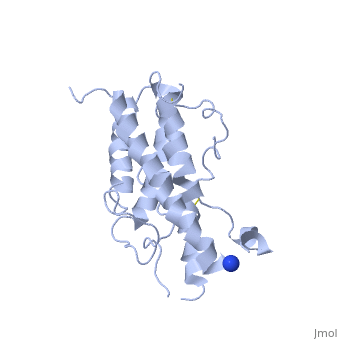
| - | <Structure load='1hgu' size='350' frame='true' align='right' caption='Representation of Somatotropin' scene=' | + | <Structure load='1hgu' size='350' frame='true' align='right' caption='Representation of Somatotropin' scene=' <scene name='86/868194/Binding_sites/1'>' /> |
The [https://en.wikipedia.org/wiki/Growth_hormone_receptor#:~:text=8%20External%20links-,Structure,GH%20binding%20protein%20(GHBP). GH membrane receptor (GHR)] is found on many cells and tissues with the exception of the brain, testicles and thymus. It is part of the [[https://en.wikipedia.org/wiki/Type_I_cytokine_receptor class I cytokine receptor family] [https://doi.org/10.1016/j.ygcen.2017.07.028 ]. The nature of this receptor is not fully understood, but it seems that it may be present in different forms due to different post-translational changes that may occur in a single protein.<ref name="m/s"> Le Cam, A. (1993), Mode d’action de l’hormone de croissance. médecine/sciences, 12:1352-61.[http://www.ipubli.inserm.fr/bitstream/handle/10608/2863/MS_1993_12_1352.pdf?sequence=1]</ref> | The [https://en.wikipedia.org/wiki/Growth_hormone_receptor#:~:text=8%20External%20links-,Structure,GH%20binding%20protein%20(GHBP). GH membrane receptor (GHR)] is found on many cells and tissues with the exception of the brain, testicles and thymus. It is part of the [[https://en.wikipedia.org/wiki/Type_I_cytokine_receptor class I cytokine receptor family] [https://doi.org/10.1016/j.ygcen.2017.07.028 ]. The nature of this receptor is not fully understood, but it seems that it may be present in different forms due to different post-translational changes that may occur in a single protein.<ref name="m/s"> Le Cam, A. (1993), Mode d’action de l’hormone de croissance. médecine/sciences, 12:1352-61.[http://www.ipubli.inserm.fr/bitstream/handle/10608/2863/MS_1993_12_1352.pdf?sequence=1]</ref> | ||
Revision as of 12:31, 22 January 2021
Somatotropin (GH for Growth Hormone or HGH for Human Growth Hormone) is a polypeptide hormone produced by the somatotropic cells of the pituitary gland. The human growth hormone complex, a protein circulating in the blood, consists of five similiar genes located over a distance of 50 kbp on the long arm of chromosome 17 [1]. There it gets encoded by the Growth hormone 1 gene along with four other related genes (hGH-N, hCS-A, hCS-B, hGH-V [1], [1])[2]. Three of these genes are encoding human chorionic somatomammotropin, which is closely related to somatotropin. They are all in the same transcriptional orientation [2]. GH is one of the best known pleiotropic hormones [3].
Functions
Somatropin plays an important role in physiological environments such as: increasing muscle mass, reducing fat mass, providing the energy necessary for tissue growth, maintaining the right level of glucose and lipids and the development of the individual's body [3]. It acts directly on a cell surface or indirectly. In the second case, somatotropin stimulates tissues such as the liver, which in turn allows the synthesis and secretion of IGF-1, thus enabling the development of cell growth, tissue, bone and thus the linear growth of the individual.[4] The GH regulates direct or indirect anabolic and growth promoting actions. Through direct regulation GH increases the amino acid uptake, the RNA- ,protein- and cartilage-synthesis and muscle growth. These regulations are often mediated by IFG. [4] However, the GH is also able to regulate catabolic actions. Thus it stimulates the breakdown of lipids (lipolysis) as is evident by increased fatty acids. A lack of GH is therefore associated with an increased lipid deposit. [5] GH can be regulated by various factors. The hypothalamus secretes hormones, like the GH releasing factor (GHR) or hormone (GHRH) which can stimulate the pituitary cells and activate different signal transduction cascades. On the other hand, it produces the hormone Somatostatin (SS) which inhibits the GH secretion by blocking the adenylate cyclase (AC). However, not the GH expression. It can also prevent the release of GHRH fom the hypthalamus. In addition, can be inhibited by feedback regulation. It stimulates the steroid and thyroid synthesis which migrate back and inhibit GH. Other regulating factors are environmental influences and the nutritional state. [6]
Structure
|
Somatotropin has three major isoforms. The predominant form is composed out of 191 amino acids and has a molecular weight of 22 kDa. The primary structure, corresponding to a sequence of amino acids, of the predominant somatotropin is the following : PUT IMAGE
Somatotropin does not exist as a linear chain of amino acids, it twists and folds on itself, forming the secondary structure. The protein, made up of a single chain, consists of four antiparallel aligned in an up-up-down-down manner [5] [7]. The first helix starts at the 6th amino acid, which is a leucine and ends with the 37th amino acid proline. It is separated from the other three helices after the 37th position. The 38th and 39th amino acids, which are lysine and glutamic acid are spliced out of the protein and therefore disconnects the first helix from the second one. The second helix starts at position 72 till 92, the third from 106 till 128 and the fourth helix from 154 until 184. All helices are ampipathic with strong , especially helix 2 is very hydrophobic. The hydrophobic protein core is usually tigthly packed and any mutations in the hidden positions lead to destablilization [1].
From the secondary structure, we obtain the tertiary structure, which corresponds to the 3D structure adopted by all the alpha helixes. The structural maintenance is stabilised by electrostatic, hydrophobic and polar interactions, and/or covalent interactions with cysteine 53 and cysteine 165 that form a as well as cysteine 182 with cysteine 189 [8]. Cys53 and C165 also link the crossover connection between helices 1 and 2 to helix 4. The other cysteine-pair form a small loop in C-terminus, involved in the receptor binding site 1 with direct contact to the extracellular domain of the GH receptor [9]. It is also requiried for stability, but unlike the first cysteine-pair not essential for biological activity [5]. A disruption of these disulphide bridges drastically reduces the molecule stability, but only if one unpaired cysteine remains intact. This may lead to implifications for diagnosis or treatments of growth disorder [10]. The protein has two different binding sites: both located at the ends of the protein, the N-terminus as well as the C-terminus [11]. The GH consists of two hydrophobic cores, one is composed of Trp104 (hGH-receptor1), Trp169 (hGH-receptor1), Pro61 (hGH), Phe176 (hGH) and Ile176 (hGH). Especially Pro61 is important, it is involved in the formation of the ative conformation of hydrophobic core amino acids and interacting with other hydrophobic core amino acids within 5Å. A mutation in this point leads to a decrease of biological and receptor binding activity. The other one is composed of two pairs of interaction between Trp76 (hGH-receptor1) and Pro48 (hGH) and between Pro106 (hGH-receptor1) and Leu45 (hGH). [1]
The second isoform was found in blood circulation and lost the amino acids 32 till 46 due to alternative splicing of the pre-mRNA and therefore has a molecular weight of 20 kDa [12]. It has a reduced insulin linked activity but is still very similiar to the predominant form, although a quarter of the amino acids in the long loop between helices 1 and 2 was deleted. The loss of these amino acids could be compensated due to the flexibility of the loop region. Through the lack of Lys41, the isoform can not form a saltbridge between hGH and the first receptor. It is possible for the hGH to compensate partially a deficit of 200Å contact surface area through Pro33 and Leu37 in hydrophobic interaction and Arg152 in salt bridge. The third isoform has a molecular weight of 17,5kDa and is formed by alternative splicing of the pre-mRNA. A mutation in the first and sixth basepair leads to a missplicing of the mRNA and loss of exon 3. The GH produced lacks amino acids 32 to 71 and causes isolated GH deficiency type II. The entire connecting loop between helices 1 and 2 and the Cys53 which is required for the first disulphide bridge is missing which leads to unpaired cysteine. Some deleted amino acids are part of a hydrophobic core which is essential to fold the molecule normally. The molecule gets instabil, it can not refold properly. Additionally, the receptor can not bind to binding site 1 which leads to a huge loss in activity. [1] [5]
HGH receptors and interactions
| |||||||||||