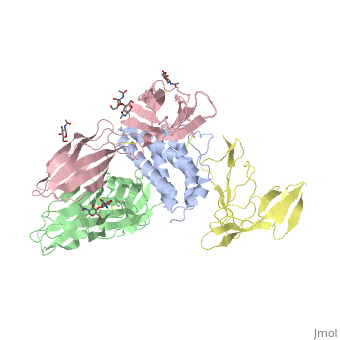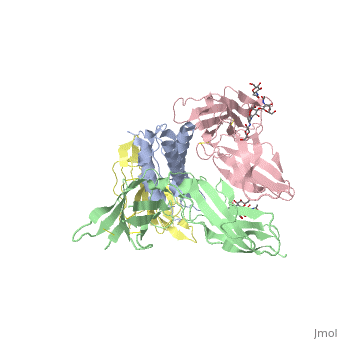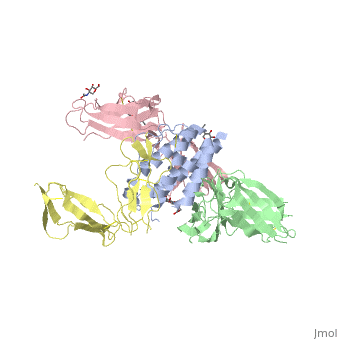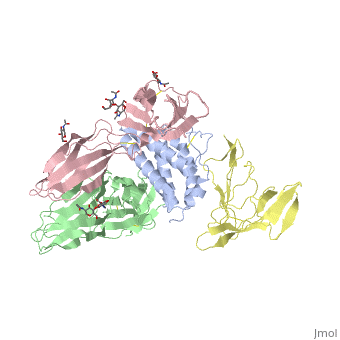2b5i
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | == | + | ==== |
| - | <StructureSection load='2b5i' size='340' side='right' caption='[[2b5i]] | + | <StructureSection load='2b5i' size='340' side='right'caption='[[2b5i]]' scene=''> |
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'> | + | <table><tr><td colspan='2'>Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id= OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol= FirstGlance]. <br> |
| - | </td></tr> | + | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2b5i FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2b5i OCA], [https://pdbe.org/2b5i PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2b5i RCSB], [https://www.ebi.ac.uk/pdbsum/2b5i PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2b5i ProSAT]</span></td></tr> |
| - | + | ||
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | |
</table> | </table> | ||
| - | == Disease == | ||
| - | [[http://www.uniprot.org/uniprot/IL2RA_HUMAN IL2RA_HUMAN]] Genetic variations in IL2RA are associated with susceptibility to diabetes mellitus insulin-dependent type 10 (IDDM10) [MIM:[http://omim.org/entry/601942 601942]]. A multifactorial disorder of glucose homeostasis that is characterized by susceptibility to ketoacidosis in the absence of insulin therapy. Clinical fetaures are polydipsia, polyphagia and polyuria which result from hyperglycemia-induced osmotic diuresis and secondary thirst. These derangements result in long-term complications that affect the eyes, kidneys, nerves, and blood vessels.<ref>PMID:17676041</ref> [[http://www.uniprot.org/uniprot/IL2_HUMAN IL2_HUMAN]] Note=A chromosomal aberration involving IL2 is found in a form of T-cell acute lymphoblastic leukemia (T-ALL). Translocation t(4;16)(q26;p13) with involves TNFRSF17. [[http://www.uniprot.org/uniprot/IL2RG_HUMAN IL2RG_HUMAN]] Defects in IL2RG are the cause of severe combined immunodeficiency X-linked T-cell-negative/B-cell-positive/NK-cell-negative (XSCID) [MIM:[http://omim.org/entry/300400 300400]]; also known as agammaglobulinemia Swiss type. A form of severe combined immunodeficiency (SCID), a genetically and clinically heterogeneous group of rare congenital disorders characterized by impairment of both humoral and cell-mediated immunity, leukopenia, and low or absent antibody levels. Patients present in infancy recurrent, persistent infections by opportunistic organisms. The common characteristic of all types of SCID is absence of T-cell-mediated cellular immunity due to a defect in T-cell development.<ref>PMID:8401490</ref> <ref>PMID:8299698</ref> <ref>PMID:8088810</ref> <ref>PMID:8027558</ref> <ref>PMID:7937790</ref> <ref>PMID:7668284</ref> <ref>PMID:7557965</ref> <ref>PMID:7860773</ref> <ref>PMID:8900089</ref> <ref>PMID:9150740</ref> Defects in IL2RG are the cause of X-linked combined immunodeficiency (XCID) [MIM:[http://omim.org/entry/312863 312863]]. XCID is a less severe form of X-linked immunodeficiency with a less severe degree of deficiency in cellular and humoral immunity than that seen in XSCID.<ref>PMID:7883965</ref> <ref>PMID:9399950</ref> | ||
| - | == Function == | ||
| - | [[http://www.uniprot.org/uniprot/IL2RA_HUMAN IL2RA_HUMAN]] Receptor for interleukin-2. [[http://www.uniprot.org/uniprot/IL2_HUMAN IL2_HUMAN]] Produced by T-cells in response to antigenic or mitogenic stimulation, this protein is required for T-cell proliferation and other activities crucial to regulation of the immune response. Can stimulate B-cells, monocytes, lymphokine-activated killer cells, natural killer cells, and glioma cells. [[http://www.uniprot.org/uniprot/IL2RG_HUMAN IL2RG_HUMAN]] Common subunit for the receptors for a variety of interleukins. [[http://www.uniprot.org/uniprot/IL2RB_HUMAN IL2RB_HUMAN]] Receptor for interleukin-2. This beta subunit is involved in receptor mediated endocytosis and transduces the mitogenic signals of IL2. | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 22: | Line 16: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2b5i ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2b5i ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | Interleukin-2 (IL-2) is an immunoregulatory cytokine that acts through a quaternary receptor signaling complex containing alpha (IL-2Ralpha), beta (IL-2Rbeta), and common gamma chain (gc) receptors. In the structure of the quaternary ectodomain complex as visualized at a resolution of 2.3 angstroms, the binding of IL-2Ralpha to IL-2 stabilizes a secondary binding site for presentation to IL-2Rbeta. gammac is then recruited to the composite surface formed by the IL-2/IL-2Rbeta complex. Consistent with its role as a shared receptor for IL-4, IL-7, IL-9, IL-15, and IL-21, gammac forms degenerate contacts with IL-2. The structure of gammac provides a rationale for loss-of-function mutations found in patients with X-linked severe combined immunodeficiency diseases (X-SCID). This complex structure provides a framework for other gammac-dependent cytokine-receptor interactions and for the engineering of improved IL-2 therapeutics. | ||
| - | |||
| - | Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors.,Wang X, Rickert M, Garcia KC Science. 2005 Nov 18;310(5751):1159-63. PMID:16293754<ref>PMID:16293754</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 2b5i" style="background-color:#fffaf0;"></div> | ||
| - | |||
| - | ==See Also== | ||
| - | *[[Interleukin|Interleukin]] | ||
| - | *[[Interleukin receptor|Interleukin receptor]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Large Structures]] |
| - | [[Category: | + | [[Category: Z-disk]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 11:26, 10 February 2021
==
| |||||||||||