Testgp
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
== G-Protein variability == | == G-Protein variability == | ||
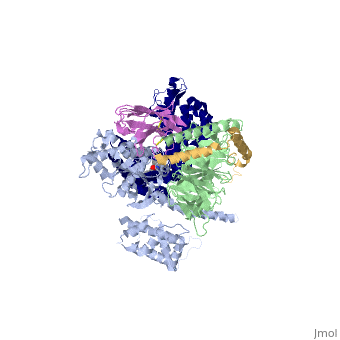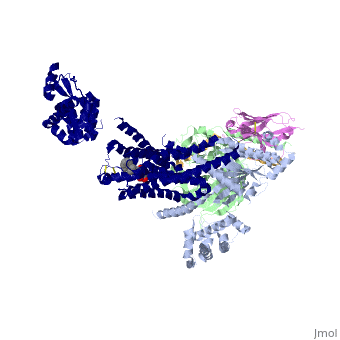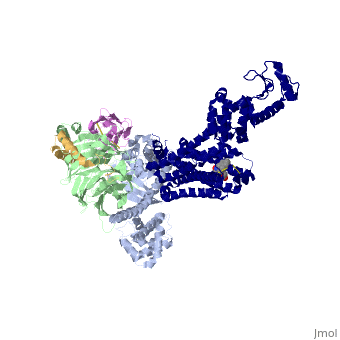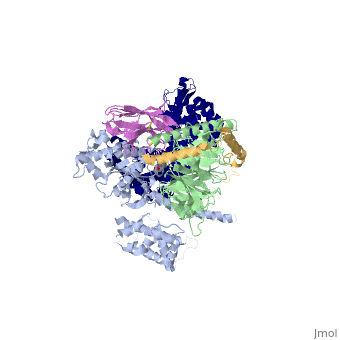
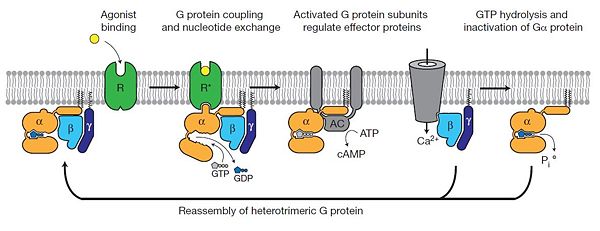
The Gαs subunit consists of two domains, the <scene name='70/701430/Alpharas/2'>Ras domain (GαsRas)</scene> and the <scene name='70/701430/Alphahelical/2'>α-helical domain (GαsAH)</scene>. A previous structure of a GTPγS bound (i.e. active, "turned on") Gαs protein (PDB ID: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1AZT 1AZT]) showed that both domains are involved in nucleotide binding, as the nucleotide-binding pocket of the Gαs subunit is formed by the interface between GαsRas and GαsAH<ref>doi:10.1126/science.278.5345.1943</ref>. It was also previously known that the GsαAH domain has a variable position relative to the GsαRas domain between this GTP bound (active) state and the nucleotide free state<ref>DOI:10.1126/science.8266082</ref><ref>doi:10.1073/pnas.1105810108</ref><ref>doi:10.1073/pnas.1113645108</ref><ref>doi:10.1038/nature10488</ref>. However, the β2AR–Gs complex structure of the receptor attached to the empty (no guanosine phosphate attached) G protein enabled comparing it to the active (GTP bound) structure and by that showing <scene name='70/701430/Gamorph/2'>how large this displacement is</scene> - this is probably the most surprising observation arising from the β2AR–Gs complex. | The Gαs subunit consists of two domains, the <scene name='70/701430/Alpharas/2'>Ras domain (GαsRas)</scene> and the <scene name='70/701430/Alphahelical/2'>α-helical domain (GαsAH)</scene>. A previous structure of a GTPγS bound (i.e. active, "turned on") Gαs protein (PDB ID: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1AZT 1AZT]) showed that both domains are involved in nucleotide binding, as the nucleotide-binding pocket of the Gαs subunit is formed by the interface between GαsRas and GαsAH<ref>doi:10.1126/science.278.5345.1943</ref>. It was also previously known that the GsαAH domain has a variable position relative to the GsαRas domain between this GTP bound (active) state and the nucleotide free state<ref>DOI:10.1126/science.8266082</ref><ref>doi:10.1073/pnas.1105810108</ref><ref>doi:10.1073/pnas.1113645108</ref><ref>doi:10.1038/nature10488</ref>. However, the β2AR–Gs complex structure of the receptor attached to the empty (no guanosine phosphate attached) G protein enabled comparing it to the active (GTP bound) structure and by that showing <scene name='70/701430/Gamorph/2'>how large this displacement is</scene> - this is probably the most surprising observation arising from the β2AR–Gs complex. | ||
| - | |||
| - | {{Template:Button Toggle Animation2}} | ||
Revision as of 15:13, 25 February 2021
| |||||||||||