User:Madison Unger/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
== Proposed Mechanism == | == Proposed Mechanism == | ||
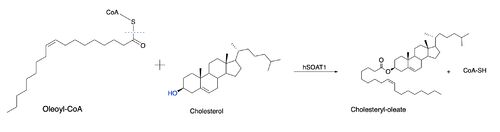
Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. However, the general mechanism involving the substrates and products of ACAT is understood. In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. [[Image:Thenewacatmech1.jpg|500px|right|thumb|Figure 2: Proposed mechanism for ACAT]] | Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. However, the general mechanism involving the substrates and products of ACAT is understood. In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. [[Image:Thenewacatmech1.jpg|500px|right|thumb|Figure 2: Proposed mechanism for ACAT]] | ||
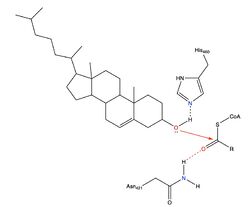
| - | However, Qian et al.<ref>PMID:32433614</ref> proposed a mechanism involving the important residues His460 and Asn421. In this mechanism, His460 acts as a general base to deprotonate the hydroxyl group on cholesterol, activating it as a [http://en.wikipedia.org/wiki/Nucleophile nucleophile]. Then, Asn421 possibly forms a hydrogen bond with oleoyl-CoA to stabilize the reaction. | + | However, Qian et al.<ref>PMID:32433614</ref> proposed a mechanism involving the important residues His460 and Asn421. [[Image:Thenewacatmech2.jpg|250px|left|thumb|Mechanism for ACAT proposed by Qian et al.]] In this mechanism, His460 acts as a general base to deprotonate the hydroxyl group on cholesterol, activating it as a [http://en.wikipedia.org/wiki/Nucleophile nucleophile]. Then, Asn421 possibly forms a hydrogen bond with oleoyl-CoA to stabilize the reaction. |
===Active Site=== | ===Active Site=== | ||
===Binding Pocket=== | ===Binding Pocket=== | ||
Revision as of 00:46, 24 March 2021
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Leah Goehring
- Gabby Smith
- Anna Campbell