We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Dustin Soe/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Function == | == Function == | ||
| - | The function of this lipase is to hydrolyze triglycerides of very low density lipoproteins (VLDL) and to aid in the delivery of lipid nutrients to vital tissues. | + | The function of this lipase is to hydrolyze triglycerides of very low density lipoproteins (VLDL) and to aid in the delivery of lipid nutrients to vital tissues. The enzyme is commonly found on the surface of cells that line blood capillaries. Two different lipoproteins are essential to break down triglycerides. One of the lipoproteins is utilized to transport fat into the bloodstream from different organs. The lipoproteins essential, in the transport of fat from the intestine are referred to as chylomicrons. VLDL are utilized in carrying triglycerides from the liver into the bloodstream. The hydrolysis of triglycerides by lipoprotein lipase results in fat molecules to be used by the body as energy or stored in fatty tissue. |
==Mechanism== | ==Mechanism== | ||
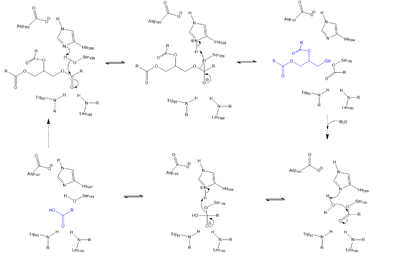
| - | + | Lipoprotein Lipase functions to catalyze the hydrolysis of one ester bond of triglycerides. It does this by utilizing a simple serine hydrolase mechanism, in which it uses a <scene name='87/877516/Catalytic_triad_master/1'>Catalytic Triad</scene> composed of Asp183, His268, and Ser159 to catalyze the hydrolysis. His268 serves as a base catalyst by deprotonating Ser159, which can then serve as the nucleophile. The transition state of the catalysis is stabilized by the <scene name='87/877516/Oxyanion_hole_master/1'>Oxyanion Hole</scene> composed of Trp82 and Leu160. The hydrolysis results in the formation of one free fatty acid and a glycerol with two fatty acid tails. | |
| - | + | [[Image:LPL_mech.png|400 px|left|thumb|Serine hydrolase mechanism utilized by LPL to catalyze the breakdown of one ester bond of a triglyceride. Compounds colored blue are the products of the hydrolysis.]] | |
== Relevance & Disease == | == Relevance & Disease == | ||
LPL is an extremely important enzyme, in that it breaks down triglycerides carried on VLDL, which leads to the reduction of cholesterol buildup. Cholesterol build up is a very serious issue with regards to obesity in the United States. In addition to this, increased plasma triglyceride levels (hypertriglyceridemia) is very unhealthy and is the leading cause of Coronary Artery Disease in America. LPL is an enzyme that helps combat this disease by breaking down the excess triglycerides that block the arteries of your heart. Very similarly, Chylomicronemia, a high level of triglycerides in the blood, causes buildup of chylomicrons (ultra-low-density lipoproteins) and leads to similar diseases. Without LPL in the body, developing coronary & metabolic (liver & pancreas) based diseases are at a higher likelihood | LPL is an extremely important enzyme, in that it breaks down triglycerides carried on VLDL, which leads to the reduction of cholesterol buildup. Cholesterol build up is a very serious issue with regards to obesity in the United States. In addition to this, increased plasma triglyceride levels (hypertriglyceridemia) is very unhealthy and is the leading cause of Coronary Artery Disease in America. LPL is an enzyme that helps combat this disease by breaking down the excess triglycerides that block the arteries of your heart. Very similarly, Chylomicronemia, a high level of triglycerides in the blood, causes buildup of chylomicrons (ultra-low-density lipoproteins) and leads to similar diseases. Without LPL in the body, developing coronary & metabolic (liver & pancreas) based diseases are at a higher likelihood | ||
Revision as of 19:35, 5 April 2021
Lipoprotein Lipase Structure
| |||||||||||