User:Madison Unger/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
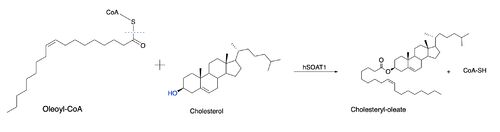
[[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | [[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | ||
| - | There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1 and ACAT2<ref>PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. | + | There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1<ref>PMID:32433614</ref> and ACAT2<ref>PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. |
== Structure == | == Structure == | ||
| Line 10: | Line 10: | ||
ACAT is a <scene name='87/877508/Overall_structure/1'>tetramer</scene> composed of a [http://en.wikipedia.org/wiki/Protein_dimer dimer] of a dimer, but is able to perform its function solely as a <scene name='87/877507/Dimer/1'>dimer</scene>. There are <scene name='87/877506/9_tm_helices_per_monomer/1'>nine transmembrane helices</scene> in each domain which create a tunnel for the active site. There are also three helices found on the intracellular side (IH1, IH2, and IH3) and one helix on the extracellular side (EH1). The active site contains three tunnels – the transmembrane tunnel for cholesterol entrance, the cytosolic tunnel for acyl-CoA entrance, and the lumen tunnel for cholesterol ester exit. ACAT also has an amino-terminal cytosolic domain (NTD) that is important for tetramerization of this protein. | ACAT is a <scene name='87/877508/Overall_structure/1'>tetramer</scene> composed of a [http://en.wikipedia.org/wiki/Protein_dimer dimer] of a dimer, but is able to perform its function solely as a <scene name='87/877507/Dimer/1'>dimer</scene>. There are <scene name='87/877506/9_tm_helices_per_monomer/1'>nine transmembrane helices</scene> in each domain which create a tunnel for the active site. There are also three helices found on the intracellular side (IH1, IH2, and IH3) and one helix on the extracellular side (EH1). The active site contains three tunnels – the transmembrane tunnel for cholesterol entrance, the cytosolic tunnel for acyl-CoA entrance, and the lumen tunnel for cholesterol ester exit. ACAT also has an amino-terminal cytosolic domain (NTD) that is important for tetramerization of this protein. | ||
=== Important Residues === | === Important Residues === | ||
| - | An important residue in the ACAT active site is His460, a | + | An important residue in the ACAT active site is His460, a Histidine, which is located in the middle of the tunnels. It is thought that His460 is located on TM7 (Qian et al.). When converting to a cholesteryl ester, the <scene name='87/877507/His460/5'>His460</scene> acts as a catalytic base that deprotonates the cholesterol. An asparagine Asn421 is another important residue in the reaction that is able to form a [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond] with acyl-CoA for stabilization. |
=== Dimer-Dimer Interactions === | === Dimer-Dimer Interactions === | ||
| - | Between the two protomers in each dimer, | + | Between the two protomers in each dimer, Van der Waals interactions occur between TM1 of one [http://en.wikipedia.org/wiki/Protomer protomer] and the lumenal TM6 and the cytosolic TM9 of the other protomer. The two dimers make limited contact within the membrane through an interface that is thought to be located between TM2, TM5, TM6 and IH2. |
== Proposed Mechanism == | == Proposed Mechanism == | ||
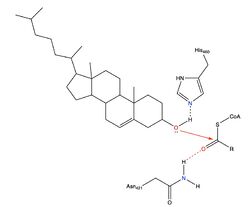
Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. However, the general mechanism involving the substrates and products of ACAT is understood<ref>PMID:32424158</ref>. [[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 2: Proposed mechanism for ACAT by Guan et al.]] In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. This mechanism is shown in Figure 2. [[Image:Thenewacatmech2.jpg|250px|right|thumb|Figure 3: Mechanism for ACAT proposed by Qian et al.]] | Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. However, the general mechanism involving the substrates and products of ACAT is understood<ref>PMID:32424158</ref>. [[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 2: Proposed mechanism for ACAT by Guan et al.]] In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. This mechanism is shown in Figure 2. [[Image:Thenewacatmech2.jpg|250px|right|thumb|Figure 3: Mechanism for ACAT proposed by Qian et al.]] | ||
Revision as of 20:05, 5 April 2021
Human Acyl-coenzyme A
| |||||||||||
References
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV Jr. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998 Oct 9;273(41):26755-64. doi: 10.1074/jbc.273.41.26755. PMID:9756919 doi:http://dx.doi.org/10.1074/jbc.273.41.26755
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Rogers MA, Liu J, Song BL, Li BL, Chang CC, Chang TY. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J Steroid Biochem Mol Biol. 2015 Jul;151:102-7. doi: 10.1016/j.jsbmb.2014.09.008., Epub 2014 Sep 12. PMID:25218443 doi:http://dx.doi.org/10.1016/j.jsbmb.2014.09.008
Student Contributors
- Leah Goehring
- Gabby Smith
- Anna Campbell