User:Megan Fleshman/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
| - | ACAT is a [[ | + | Acyl-Coenzyme A Cholesterol Acyltransferase (ACAT), or also known as Sterol ''O''-Acyltransferase (SOAT), is an important enzyme in the body. |
| + | Cholesterol esters were found in arterial lesions in 1910, but the first ACAT activity was discovered in the mid 1900's. This led to the inhibition of ACAT as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT inhibitors grew, but some of the compounds looked at exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT gene was successfully cloned. This discovery led to more studies with ACAT and atherosclerosis. Some of these studies used mice and showed cellular toxicity. ACAT inhibition is still being looked into as a strategy for treatment or prevention of atherosclerosis and related diseases. | ||
| + | <ref name=”Farese Jr.”>PMID: 16857957</ref> | ||
| + | [[Image:Screen Shot 2021-03-16 at 3.11.39 PM.png|400 px|right|thumb|Figure 1. ACAT as a Dimer of Dimers - One Monomer is Highlighted]] | ||
==Mechanism== | ==Mechanism== | ||
| Line 14: | Line 17: | ||
The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel. | The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel. | ||
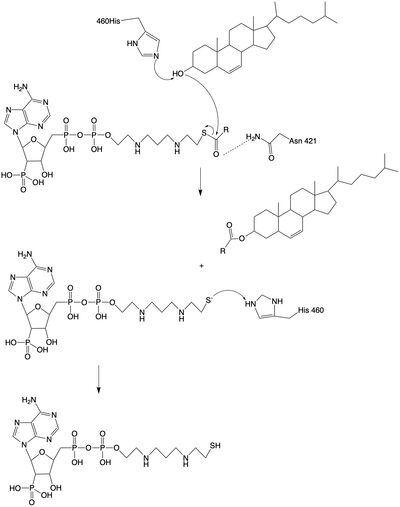
| - | The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure | + | The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure 2). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol. |
| - | [[Image:acatmechanism.jpg|400px|left|thumb|Figure | + | [[Image:acatmechanism.jpg|400px|left|thumb|Figure 2: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.]] |
Crystal Structure of the Entamoeba histolytica RNA lariat debranching enzyme. <ref name=”Ransey”>PMID:28504306</ref> | Crystal Structure of the Entamoeba histolytica RNA lariat debranching enzyme. <ref name=”Ransey”>PMID:28504306</ref> | ||
Revision as of 23:38, 5 April 2021
| |||||||||||
References
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
Student Contributors
- Megan Fleshman, Tori Templin, Haylie Moehlenkamp