User:Megan Fleshman/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
<ref name=”Farese Jr.”>PMID: 16857957</ref> | <ref name=”Farese Jr.”>PMID: 16857957</ref> | ||
[[Image:Screen Shot 2021-03-16 at 3.11.39 PM.png|400 px|right|thumb|Figure 1. ACAT as a Dimer of Dimers - One Monomer is Highlighted]] | [[Image:Screen Shot 2021-03-16 at 3.11.39 PM.png|400 px|right|thumb|Figure 1. ACAT as a Dimer of Dimers - One Monomer is Highlighted]] | ||
| + | |||
| + | |||
| + | == Structural highlights == | ||
| + | ACAT is a dimer of dimers, which is also known as a [https://en.wikipedia.org/wiki/Tetramer tetramer]. | ||
| + | This <scene name='87/877604/Tetramer/2'>tetramer</scene> is about 260 kDa and is composed completely of helices, with each monomer containing 9 transmembrane helices, which have been color-coordinated to help with orientation within structures. The <scene name='87/877604/Colored_dimer/3'>dimer of ACAT</scene> was found to be the active arrangement. | ||
| + | The <scene name='87/877604/Dimer_interface/1'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway. The cholesterol enters through the T tunnel while the acyl-CoA enters through the C tunnel. The reaction is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol from the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen. | ||
==Mechanism== | ==Mechanism== | ||
| Line 19: | Line 25: | ||
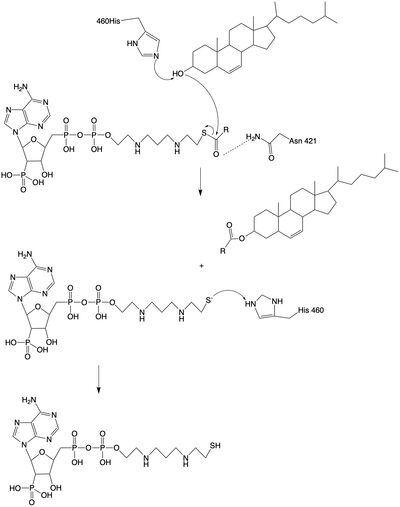
The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure 2). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol. | The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure 2). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol. | ||
[[Image:acatmechanism.jpg|400px|left|thumb|Figure 2: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.]] | [[Image:acatmechanism.jpg|400px|left|thumb|Figure 2: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.]] | ||
| - | |||
| - | Crystal Structure of the Entamoeba histolytica RNA lariat debranching enzyme. <ref name=”Ransey”>PMID:28504306</ref> | ||
| - | |||
| - | == Function == | ||
| - | ACAT article <ref name=”Qian”>PMID:32433614</ref> | ||
| - | SOAT Article <ref name=”Shengcheng”>PMID:32424158</ref> | ||
| - | |||
| - | == Disease == | ||
| - | |||
| - | == Relevance == | ||
| - | |||
| - | == Structural highlights == | ||
| - | ACAT is a dimer of dimers, which is also known as a [https://en.wikipedia.org/wiki/Tetramer tetramer]. | ||
| - | This <scene name='87/877604/Tetramer/2'>tetramer</scene> is about 260 kDa and is composed completely of helices, with each monomer containing 9 transmembrane helices, which have been color-coordinated to help with orientation within structures. The <scene name='87/877604/Colored_dimer/3'>dimer of ACAT</scene> was found to be the active arrangement. | ||
| - | The <scene name='87/877604/Dimer_interface/1'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway. The cholesterol enters through the T tunnel while the acyl-CoA enters through the C tunnel. The reaction is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol from the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen. | ||
Revision as of 23:42, 5 April 2021
| |||||||||||
References
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
Student Contributors
- Megan Fleshman, Tori Templin, Haylie Moehlenkamp