Introduction
Acyl-Coenzyme A Cholesterol Acyltransferase (ACAT), or also known as Sterol O-Acyltransferase (SOAT), is an important enzyme in the body.
Cholesterol esters were found in arterial lesions in 1910, but the first ACAT activity was discovered in the mid 1900's. This led to the inhibition of ACAT as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT inhibitors grew, but some of the compounds looked at exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT gene was successfully cloned. This discovery led to more studies with ACAT and atherosclerosis. Some of these studies used mice and showed cellular toxicity. ACAT inhibition is still being looked into as a strategy for treatment or prevention of atherosclerosis and related diseases.
[1]

Figure 1. ACAT as a Dimer of Dimers - One Monomer is Highlighted
Structural highlights
ACAT is a dimer of dimers, which is also known as a tetramer.
This is about 260 kDa and is composed completely of helices, with each monomer containing 9 transmembrane helices, which have been color-coordinated to help with orientation within structures. The was found to be the active arrangement.
The is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway. The cholesterol enters through the T tunnel while the acyl-CoA enters through the C tunnel. The reaction is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol from the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen.
Mechanism
Tunnels
The catalytic site is accessed through three different tunnels that lead from the center catalytic domain of the monomer, to the [lumen], cytosol, and transmembrane space. The tunnels allow the entrance of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function.
The is open to the cytosolic side of the protein in which the Acyl CoA enters into the catalytic domain.
The catalytic site contains conserved that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid tail.
The is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important of the T tunnel include Arginine262, Phenylalanine 263, and Leucine 306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism.
The is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel.
The mechanism of the [acyltransferace]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure 2). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol.
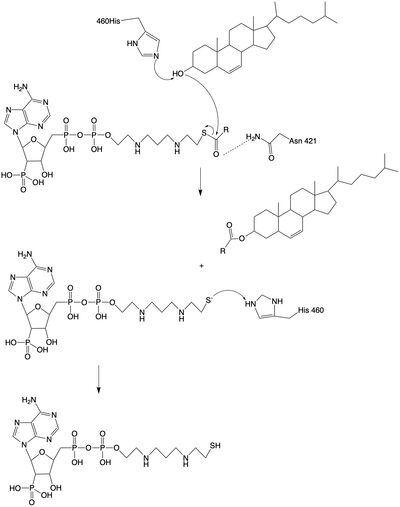
Figure 2: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.


