We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
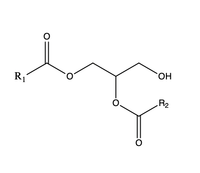
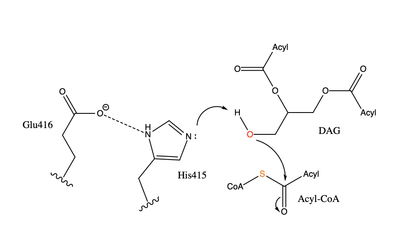
Diacylglycerol acyltransferase (DGAT) catalyzes the final and only committed step of triacylglycerol synthesis <ref name="Cases">PMID:9789033</ref>. It does this by using diacylglycerol (DAG) and oleoyl CoA as substrates. DGAT plays a pivotal role in the metabolism of DAG and is important to the process of triacylglycerol metabolism. This metabolism is involved in intestinal fat absorption, lipoprotein assembly, lactation, and adipose tissue formation <ref name="Yen">PMID:18757836</ref>. | Diacylglycerol acyltransferase (DGAT) catalyzes the final and only committed step of triacylglycerol synthesis <ref name="Cases">PMID:9789033</ref>. It does this by using diacylglycerol (DAG) and oleoyl CoA as substrates. DGAT plays a pivotal role in the metabolism of DAG and is important to the process of triacylglycerol metabolism. This metabolism is involved in intestinal fat absorption, lipoprotein assembly, lactation, and adipose tissue formation <ref name="Yen">PMID:18757836</ref>. | ||
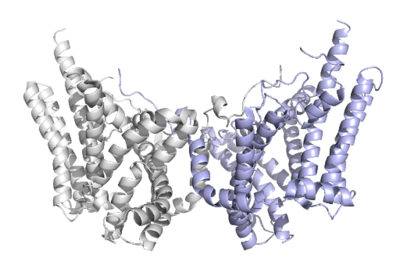
DGAT1 is a [http://en.wikipedia.org/wiki/Membrane_protein membrane protein] responsible for the conversion of diacylglycerols to triacylglycerols. | DGAT1 is a [http://en.wikipedia.org/wiki/Membrane_protein membrane protein] responsible for the conversion of diacylglycerols to triacylglycerols. | ||
| - | |||
| - | == Disease == | ||
| - | |||
| - | == Biological Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 13:14, 6 April 2021
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Sui, X., Wang, K., Gluchowski, N. L., Elliott, S. D., Liao, M., Walther, T. C., & Farese, R. V. (2020). Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature, 581(7808), 323-328. doi:10.1038/s41586-020-2289-6
- ↑ Wang L;Qian H;Nian Y;Han Y;Ren Z;Zhang H;Hu L;Prasad BVV;Laganowsky A;Yan N;Zhou M;. (2020, May 13). Structure and mechanism of human diacylglycerol o-acyltransferase 1. Retrieved March 09, 2021, from https://pubmed.ncbi.nlm.nih.gov/32433610/
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko