User:Megan Fleshman/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
The catalytic site is accessed through three different tunnels that lead from the center catalytic domain of the monomer, to the [[http://en.wikipedia.org/wiki/Lumen_(anatomy) lumen]], cytosol, and transmembrane space. The tunnels allow the entrance of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function. | The catalytic site is accessed through three different tunnels that lead from the center catalytic domain of the monomer, to the [[http://en.wikipedia.org/wiki/Lumen_(anatomy) lumen]], cytosol, and transmembrane space. The tunnels allow the entrance of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function. | ||
The <scene name='87/877605/C_tunnel/1'>C tunnel</scene> is open to the cytosolic side of the protein in which the Acyl CoA enters into the catalytic domain. | The <scene name='87/877605/C_tunnel/1'>C tunnel</scene> is open to the cytosolic side of the protein in which the Acyl CoA enters into the catalytic domain. | ||
| + | The <scene name='87/877605/T_tunnel/1'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important <scene name='87/877605/T_tunnel_residues/1'>residues</scene> of the T tunnel include Arginine262, Phenylalanine 263, and Leucine 306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism. | ||
| + | The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel. | ||
| Line 25: | Line 27: | ||
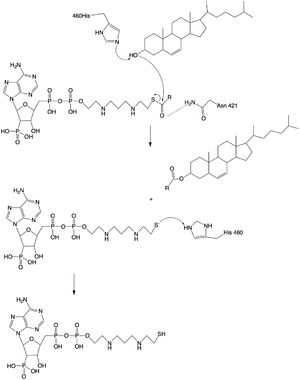
The catalytic site contains conserved <scene name='87/877605/Catalytic_residues/1'>residues</scene> that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid tail. | The catalytic site contains conserved <scene name='87/877605/Catalytic_residues/1'>residues</scene> that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid tail. | ||
| - | The <scene name='87/877605/T_tunnel/1'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important <scene name='87/877605/T_tunnel_residues/1'>residues</scene> of the T tunnel include Arginine262, Phenylalanine 263, and Leucine 306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism. | ||
| - | The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel. | ||
==Mechanism== | ==Mechanism== | ||
Revision as of 18:42, 6 April 2021
| |||||||||||
References
ACAT article [3] SOAT Article [4]
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Chang TY, Chang CC, Bryleva E, Rogers MA, Murphy SR. Neuronal cholesterol esterification by ACAT1 in Alzheimer's disease. IUBMB Life. 2010 Apr;62(4):261-7. doi: 10.1002/iub.305. PMID:20101629 doi:http://dx.doi.org/10.1002/iub.305
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
Student Contributors
- Megan Fleshman, Tori Templin, Haylie Moehlenkamp