User:Madison Unger/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
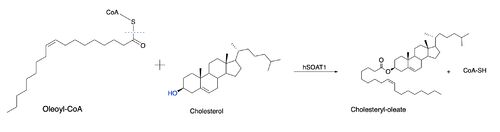
[[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | [[Image:Thenewacatmech1.jpg|500px|left|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | ||
| - | There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1<ref>PMID:32433614</ref> and ACAT2<ref>PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. | + | There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1<ref name="Quin">PMID:32433614</ref> and ACAT2<ref name="Cases">PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. |
== Structure == | == Structure == | ||
| Line 14: | Line 14: | ||
Between the two protomers in each dimer, Van der Waals interactions occur between TM1 of one [http://en.wikipedia.org/wiki/Protomer protomer] and the lumenal TM6 and the cytosolic TM9 of the other protomer. The two dimers make limited contact within the membrane through an interface that is thought to be located between TM2, TM5, TM6 and IH2. | Between the two protomers in each dimer, Van der Waals interactions occur between TM1 of one [http://en.wikipedia.org/wiki/Protomer protomer] and the lumenal TM6 and the cytosolic TM9 of the other protomer. The two dimers make limited contact within the membrane through an interface that is thought to be located between TM2, TM5, TM6 and IH2. | ||
== Proposed Mechanism == | == Proposed Mechanism == | ||
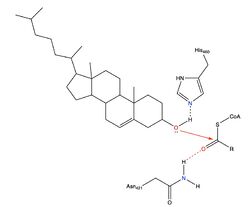
| - | Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. [[Image:Thenewacatmech2.jpg|250px|left|thumb|Figure 3: Mechanism for ACAT proposed by Qian et al.]] However, the general mechanism involving the substrates and products of ACAT is understood<ref>PMID:32424158</ref>. In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. | + | Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. [[Image:Thenewacatmech2.jpg|250px|left|thumb|Figure 3: Mechanism for ACAT proposed by Qian et al.]] However, the general mechanism involving the substrates and products of ACAT is understood<ref name="Guan">PMID:32424158</ref>. In this reaction, [http://en.wikipedia.org/wiki/Stearoyl-CoA_9-desaturase oleoyl-CoA] and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the [http://en.wikipedia.org/wiki/Thioester thioester] bond of oleoyl-CoA, kicking off CoA-SH as a leaving group. |
However, Qian et al.<ref>PMID:32433614</ref> proposed a mechanism involving the important residues His460 and Asn421. In this mechanism, His460 acts as a general base to deprotonate the hydroxyl group on cholesterol, activating it as a [http://en.wikipedia.org/wiki/Nucleophile nucleophile]. Then, Asn421 possibly forms a hydrogen bond with oleoyl-CoA to stabilize the reaction (Fig. 3). | However, Qian et al.<ref>PMID:32433614</ref> proposed a mechanism involving the important residues His460 and Asn421. In this mechanism, His460 acts as a general base to deprotonate the hydroxyl group on cholesterol, activating it as a [http://en.wikipedia.org/wiki/Nucleophile nucleophile]. Then, Asn421 possibly forms a hydrogen bond with oleoyl-CoA to stabilize the reaction (Fig. 3). | ||
===Active Site=== | ===Active Site=== | ||
Revision as of 19:03, 12 April 2021
Human Acyl-CoenzymeA
| |||||||||||
References
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV Jr. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998 Oct 9;273(41):26755-64. doi: 10.1074/jbc.273.41.26755. PMID:9756919 doi:http://dx.doi.org/10.1074/jbc.273.41.26755
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Rogers MA, Liu J, Song BL, Li BL, Chang CC, Chang TY. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J Steroid Biochem Mol Biol. 2015 Jul;151:102-7. doi: 10.1016/j.jsbmb.2014.09.008., Epub 2014 Sep 12. PMID:25218443 doi:http://dx.doi.org/10.1016/j.jsbmb.2014.09.008
- ↑ Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer's disease: the lipid connection. J Neurochem. 2007 Nov;103 Suppl 1:159-70. doi: 10.1111/j.1471-4159.2007.04715.x. PMID:17986151 doi:http://dx.doi.org/10.1111/j.1471-4159.2007.04715.x
- ↑ Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S, Konieczny SF, Ratliff TL, Liu X, Xie J, Cheng JX. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016 Dec 15;35(50):6378-6388. doi: 10.1038/onc.2016.168. Epub 2016 May , 2. PMID:27132508 doi:http://dx.doi.org/10.1038/onc.2016.168
- ↑ Rudel LL, Shelness GS. Cholesterol esters and atherosclerosis-a game of ACAT and mouse. Nat Med. 2000 Dec;6(12):1313-4. doi: 10.1038/82110. PMID:11100106 doi:http://dx.doi.org/10.1038/82110
Student Contributors
- Leah Goehring
- Gabby Smith
- Anna Campbell