This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Dustin Soe/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
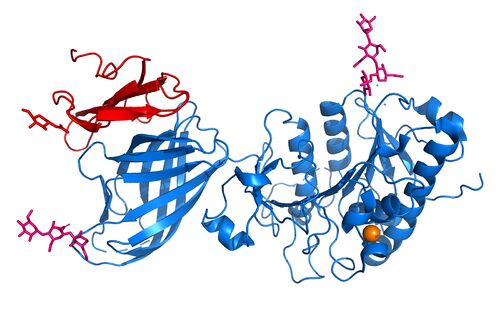
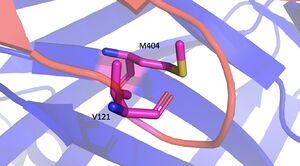
LPL is an extremely important enzyme, in that it breaks down triglycerides carried on VLDL, which leads to the reduction of cholesterol buildup. Cholesterol build up is a very serious issue with regards to obesity in the United States. In addition to this, increased plasma triglyceride levels (hypertriglyceridemia) is very unhealthy and is the leading cause of Coronary Artery Disease in America. LPL is an enzyme that helps combat this disease by breaking down the excess triglycerides that block the arteries of your heart. Very similarly, Chylomicronemia, a high level of triglycerides in the blood, causes buildup of chylomicrons (ultra-low-density lipoproteins) and leads to similar diseases. Without LPL in the body, developing coronary & metabolic (liver & pancreas) based diseases are at a higher likelihood. [[Image:M404_No_Mutation_copy.jpg|300 px|right|thumb|No Mutation Image]] | LPL is an extremely important enzyme, in that it breaks down triglycerides carried on VLDL, which leads to the reduction of cholesterol buildup. Cholesterol build up is a very serious issue with regards to obesity in the United States. In addition to this, increased plasma triglyceride levels (hypertriglyceridemia) is very unhealthy and is the leading cause of Coronary Artery Disease in America. LPL is an enzyme that helps combat this disease by breaking down the excess triglycerides that block the arteries of your heart. Very similarly, Chylomicronemia, a high level of triglycerides in the blood, causes buildup of chylomicrons (ultra-low-density lipoproteins) and leads to similar diseases. Without LPL in the body, developing coronary & metabolic (liver & pancreas) based diseases are at a higher likelihood. [[Image:M404_No_Mutation_copy.jpg|300 px|right|thumb|No Mutation Image]] | ||
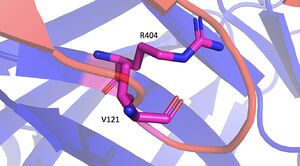
[[Image:M404ToR404Pymol_copy_2.jpg|300 px|right|thumb|Mutation Image]] | [[Image:M404ToR404Pymol_copy_2.jpg|300 px|right|thumb|Mutation Image]] | ||
| + | |||
| + | <ref name=”Arora”>PMID:31072929</ref> | ||
| + | <ref name=”Birrane”>PMID:30559189</ref> | ||
| + | <ref name=”Davies”>PMID:20620994</ref> | ||
| + | <ref name=”Beigneux”>PMID:30850549</ref> | ||
== Structural highlights == | == Structural highlights == | ||
| Line 19: | Line 24: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | |||
==Student Contributors== | ==Student Contributors== | ||
Revision as of 19:16, 12 April 2021
Lipoprotein Lipase (LPL)
| |||||||||||