We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Induced fit
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
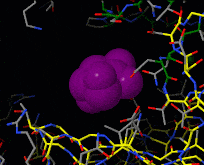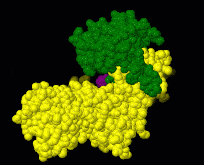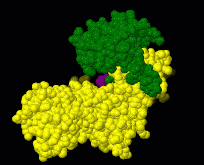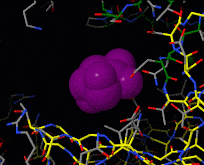
| - | [[Image:Induced fit.gif|frame|Morph of hexokinase in the open [[ | + | [[Image:Induced fit.gif|frame|Morph of hexokinase in the open [[3o80]] and glucose-bound closed [[3o8m]] conformation. For reference, glucose (purple) is shown throughout the morph. Two views are shown, an overview as spacefill and a detail of the binding site in wireframe.]] |
[[Induced fit]] describes a conformational change in a protein when it binds a ligand, in contrast to a lock-and-key model of ligand binding. A classical example of induced fit is binding of glucose to hexokinase, depicted in a morph between [[3o8m]] and [[3o80]] below. | [[Induced fit]] describes a conformational change in a protein when it binds a ligand, in contrast to a lock-and-key model of ligand binding. A classical example of induced fit is binding of glucose to hexokinase, depicted in a morph between [[3o8m]] and [[3o80]] below. | ||
Revision as of 15:36, 13 April 2021
Induced fit describes a conformational change in a protein when it binds a ligand, in contrast to a lock-and-key model of ligand binding. A classical example of induced fit is binding of glucose to hexokinase, depicted in a morph between 3o8m and 3o80 below.
Contents |
History of the concept
Induced fit was suggested by Koshland in 1958 [1], providing an alternative to the lock-and-key binding model that Emil Fischer proposed in 1899 [2].
Interactive examples
| |||||||||||
See Also
- A morph of induced fit when Tamiflu binds to Neuraminidase.