User:Haylie Moehlenkamp/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
==Inhibitor== | ==Inhibitor== | ||
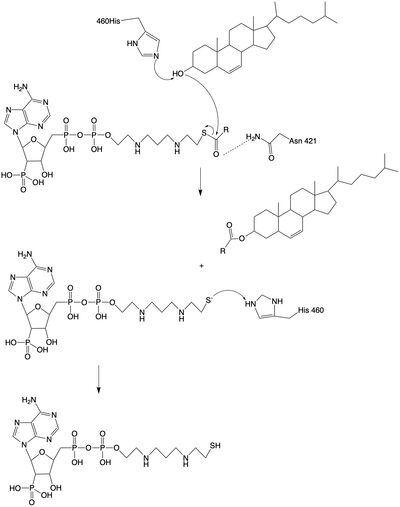
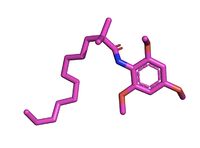
| - | The <scene name='87/877626/Overlay/10'>overlay</scene> illustrates the competitive inhibition of Acetyl-CoA and the inhibitor CI-976. Structurally, Acetyl-CoA and CI-976 are both largely hydrophobic, each with long hydrophobic tails and aromatic heads. As evident in this image, the hydrophobic tail of CI-976, mimics that of Acetyl-CoA. This allows for the inhibitor to be recognized by ACAT and to bind tightly in the active site pocket, blocking Acetyl-CoA from binding, thus rendering ACAT unable to perform its reaction. [[Image: CI-976-2.jpg|200 px|right|thumb|Figure 3. CI-976 Inhibitor]] | + | The <scene name='87/877626/Overlay/10'>overlay</scene> illustrates the competitive inhibition of Acetyl-CoA and the inhibitor CI-976. According to Guan and colleagues, there have been a number of iterations of ACAT inhibitors have been created, and many of them of different structure formations <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref>. CI-976 specifically is known as a small molecule inhibitor that is part of what is called the fatty acyl amide analog family, and functions as a competitive inhibitor of Acyl Co-A <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref>. Guan discussed that this inhibitor in previous studies had shown that CI-976 reduced the size of atherosclerotic plaques and cholesterol levels in plasma <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref> |
| + | Structurally, Acetyl-CoA and CI-976 are both largely hydrophobic, each with long hydrophobic tails and aromatic heads. As evident in this image, the hydrophobic tail of CI-976, mimics that of Acetyl-CoA. This allows for the inhibitor to be recognized by ACAT and to bind tightly in the active site pocket, blocking Acetyl-CoA from binding, thus rendering ACAT unable to perform its reaction. [[Image: CI-976-2.jpg|200 px|right|thumb|Figure 3. CI-976 Inhibitor]] | ||
| Line 44: | Line 45: | ||
===Other Diseases=== | ===Other Diseases=== | ||
| - | ACAT is also involved in diseases such as [https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055 Parkinson’s Disease] and other neurodegenerative diseases due to the accumulation of Aβ plaques in the brain. After research on [https://www.mayoclinic.org/diseases-conditions/glioma/symptoms-causes/syc-20350251 glioma], [https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 prostate cancer], [https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 pancreatic cancer], [https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 leukemia], and [https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470 breast cancer], it has been noted that ACAT plays a role in the progression of cancer over time. Recently, Ayyagari et al. found that there was a significant increase in ACAT-1 expression in ovarian cancer cell lines <ref name="Ayyagari"> doi:10.1371/journal.pone.0228024</ref>. ACAT-2 is believed to be upregulated in Nephrotic Syndrome (NS) which can lead to cardiovascular disease and renal diseases <ref name="Vaziri"> doi:10.1161/01.CIR.0000136023.70841.0F</ref>. Because of ACAT's activity in tissues such as the aorta, intestine, and liver, it plays a role in Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Studies have shown that the inhibition of ACAT-2 can slow the progression of Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. | + | ACAT is also involved in diseases such as [https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055 Parkinson’s Disease] and other neurodegenerative diseases due to the accumulation of Aβ plaques in the brain. After research on [https://www.mayoclinic.org/diseases-conditions/glioma/symptoms-causes/syc-20350251 glioma], [https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 prostate cancer], [https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 pancreatic cancer], [https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 leukemia], and [https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470 breast cancer], it has been noted that ACAT plays a role in the progression of cancer over time. Recently, Ayyagari et al. found that there was a significant increase in ACAT-1 expression in ovarian cancer cell lines <ref name="Ayyagari"> doi:10.1371/journal.pone.0228024</ref>. ACAT-2 is believed to be upregulated in Nephrotic Syndrome (NS) which can lead to cardiovascular disease and renal diseases <ref name="Vaziri"> doi:10.1161/01.CIR.0000136023.70841.0F</ref>. Because of ACAT's activity in tissues such as the aorta, intestine, and liver, it plays a role in Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Studies have shown that the inhibition of ACAT-2 can slow the progression of Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Guan discussed a previous study which found that CI-976 decreased the size of atherosclerosis plaques and the overall concentration of cholesterol in the blood plasma of animals that had been fed a high cholesterol diet <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref>. |
| - | + | ||
| - | <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref> | + | |
Revision as of 19:09, 13 April 2021
| |||||||||||
References
ACAT article [9] SOAT Article [10]
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ 3.0 3.1 3.2 3.3 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 4.0 4.1 4.2 4.3 4.4 Chang TY, Chang CC, Bryleva E, Rogers MA, Murphy SR. Neuronal cholesterol esterification by ACAT1 in Alzheimer's disease. IUBMB Life. 2010 Apr;62(4):261-7. doi: 10.1002/iub.305. PMID:20101629 doi:http://dx.doi.org/10.1002/iub.305
- ↑ 5.0 5.1 5.2 5.3 5.4 Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. PMID:26669800 doi:http://dx.doi.org/10.4155/fmc.15.161
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
- ↑ Vaziri ND, Liang KH. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004 Jul 27;110(4):419-25. doi: 10.1161/01.CIR.0000136023.70841.0F. , Epub 2004 Jul 19. PMID:15262831 doi:http://dx.doi.org/10.1161/01.CIR.0000136023.70841.0F
- ↑ 8.0 8.1 Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1262-7. doi: 10.1073/pnas.0336398100., Epub 2003 Jan 21. PMID:12538880 doi:http://dx.doi.org/10.1073/pnas.0336398100
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
Student Contributors
- Haylie Moehlenkamp, Tori Templin, Megan Fleshman