This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Leanne Price/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
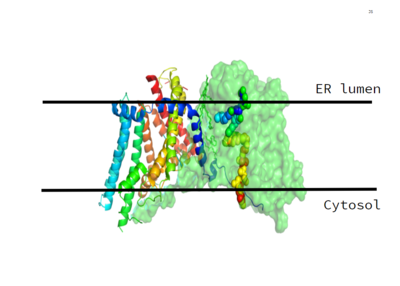
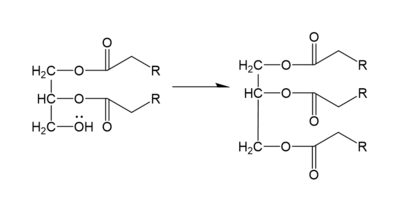
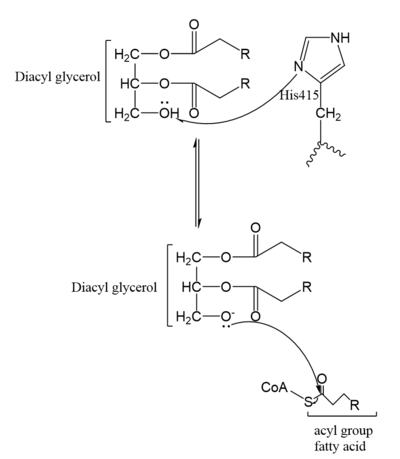
DGAT consists of two domains, one cytoplasmic and one luminal. The cytoplasmic domain interacts with the interior of the cell and relays signals. The luminal domain senses misfolded proteins. The structure of DGAT consists of two protein chains, one ligand, two polymers, eighteen <scene name='87/877601/Transmembrane_helices/2'>alpha helices</scene> and zero beta sheets. The transmembrane helices form a large central cavity within the membrane that opens to the bilayer via a wide lateral gate. Through openings on the cytosolic and luminal sides of DGAT, this central cavity is also accessible. The majority of the transmembrane helices present within the structure also form a concave-shaped ridge on either side of the membrane. These aspects of the domain structure are deemed as the 'MBOAT core'. Within this core, a tunnel-like region, similar to a binding pocket, is also present. Access to the active site of DGAT by substrates is done through the lateral gate, which lies on the ER lumen side, within the membrane. This tunnel-like region is referred to as the cytosolic, or C, tunnel. <ref name="Sui" /> | DGAT consists of two domains, one cytoplasmic and one luminal. The cytoplasmic domain interacts with the interior of the cell and relays signals. The luminal domain senses misfolded proteins. The structure of DGAT consists of two protein chains, one ligand, two polymers, eighteen <scene name='87/877601/Transmembrane_helices/2'>alpha helices</scene> and zero beta sheets. The transmembrane helices form a large central cavity within the membrane that opens to the bilayer via a wide lateral gate. Through openings on the cytosolic and luminal sides of DGAT, this central cavity is also accessible. The majority of the transmembrane helices present within the structure also form a concave-shaped ridge on either side of the membrane. These aspects of the domain structure are deemed as the 'MBOAT core'. Within this core, a tunnel-like region, similar to a binding pocket, is also present. Access to the active site of DGAT by substrates is done through the lateral gate, which lies on the ER lumen side, within the membrane. This tunnel-like region is referred to as the cytosolic, or C, tunnel. <ref name="Sui" /> | ||
| - | [[Image:Access.JPG|400 px|left|thumb|Figure 3. Shows the location of the lateral gate accessed via the ER lumen side of the membrane in pink, with the cytosolic tunnel shown in the back | + | [[Image:Access.JPG|400 px|left|thumb|Figure 3. Shows the location of the lateral gate accessed via the ER lumen side of the membrane in pink, with the substrate acyl-CoA in the cytosolic tunnel (red) shown in the back. ]] |
Revision as of 20:00, 20 April 2021
Diacylglycerol Acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 2.0 2.1 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ https://www.proteinatlas.org/ENSG00000185000-DGAT1/pathology
Student Contributors
- Justin Smith
- Eloi Bigirimana
- Leanne Price