We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='6VYI' size='350' frame='true' side='right' caption='Human Diacylglycerol O-Transferase 1 6VYI' scene=’His415 in Active Site’> | <StructureSection load='6VYI' size='350' frame='true' side='right' caption='Human Diacylglycerol O-Transferase 1 6VYI' scene=’His415 in Active Site’> | ||
== Introduction == | == Introduction == | ||
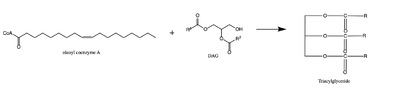
| - | [[Image: | + | [[Image:dag rxn.png|400 px|right|thumb|Figure 1: Reaction of oleoyl-CoA with DAG to produce a triglyceride]] |
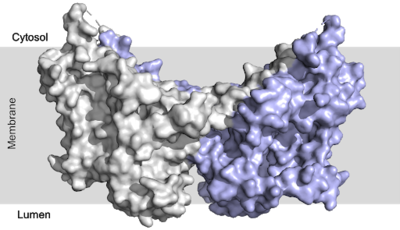
| - | There are two families of DGAT proteins each with their own distinct cellular functions via synthesis of triacylglycerides from oleoyl-CoA. Diacylglycerol acyltransferase 1 (DGAT1) catalyzes the final and only committed step of [https://en.wikipedia.org/wiki/Triglyceride triacylclygerol synthesis] (Fig. | + | There are two families of DGAT proteins each with their own distinct cellular functions via synthesis of triacylglycerides from oleoyl-CoA. Diacylglycerol acyltransferase 1 (DGAT1) catalyzes the final and only committed step of [https://en.wikipedia.org/wiki/Triglyceride triacylclygerol synthesis] (Fig. 1). <ref name="Cases">PMID:9789033</ref> It does this by using diacylglycerol (DAG) and oleoyl CoA as substrates. DGAT1 is located in the membrane of the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum] and is important for metabolism through its uptake of diacylglycerides and synthesis of triacylglicerides (Fig. 2). <ref name="Sui">PMID:32433611</ref> This metabolism is involved in intestinal fat absorption, lipoprotein assembly, lactation, and adipose tissue formation <ref name="Yen">PMID:18757836</ref>. |
| - | [[Image: | + | [[Image:dgat with domains.png|400 px|right|thumb|Figure 2: DGAT1 with cytosolic, transmembrane, and luminal domains]] |
== Structural highlights == | == Structural highlights == | ||
| Line 20: | Line 20: | ||
=== Mechanism === | === Mechanism === | ||
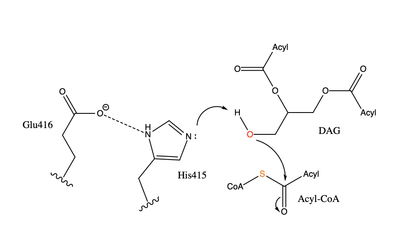
| - | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4]] When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, DGAT1 transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride (Fig. | + | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4]] When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, DGAT1 transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride (Fig. 1). The <scene name='87/878228/415_416_465_with_oleoyl_coa/2'>Catalytic His415</scene> deprotonates the hydroxyl group on the C3 of the glycerol backbone. The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and the tetrahedral acyl-CoA-DAG intermediate is formed which is likely stabilized by Gln465. <ref name="Wang">PMID:32433610</ref>The electron density then falls back down to the carbonyl carbon and to the sulfur of the Acyl-CoA which accepts the added electron density and the bond between the sulfur and carbonyl carbon is broken. Glu416 likely provides enhancement of the reaction by deprotonating His415 which shifts the electron density and helps facilitate the deprotonation by His415 of the glycerol backbone despite being outside of the 3 angstrom hydrogen bonding distance. This is likely due to the fact that the DGAT 1 enzyme was unable to be visualized with the diacylglycerol in the active site. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 and Glu416 support the hypothesis that it is essential for catalysis in the active site since the enzyme function was completely eliminated when the mutation was made. <ref name="Wang">PMID:32433610</ref> |
===Inhibitors=== | ===Inhibitors=== | ||
Revision as of 02:58, 23 April 2021
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
- ↑ 6.0 6.1 Gluchowski, N. L., Chitraju, C., Picoraro, J. A., Mejhert, N., Pinto, S., Xin, W., Kamin, D. S., Winter, H. S., Chung, W. K., Walther, T. C., & Farese, R. V., Jr (2017). Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. Journal of lipid research, 58(6), 1230–1237. https://doi.org/10.1194/jlr.P075119
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko