We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Kaitlyn Roberts/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=== Tunnel System === | === Tunnel System === | ||
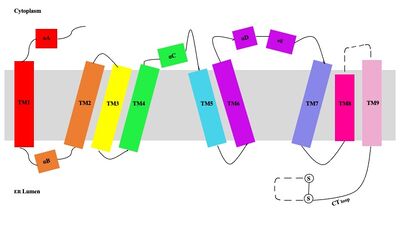
| - | A main structural element of this enzyme is the tunnel systems. [[Image:Tunnels2.jpg|350 px|right|thumb|'''Figure 4.''' | + | A main structural element of this enzyme is the tunnel systems. [[Image:Tunnels2.jpg|350 px|right|thumb|'''Figure 4.''' 2D layout of the SOAT tunnel system. The orientation of the tunnels shows the C tunnel opening to the cytosol and the L tunnel opening to the lumen. The T tunnel opens into the membrane, but is not quite the 90 degree shown in the 2D image.]] There are 3 main tunnels in each monomer: the cytosolic (C) tunnel opening to the cytosol, the transmembrane(T) tunnel opening to the membrane, and the lumenal (L) tunnel opens to the lumen. The C tunnel opens to the cytosol of the cell and is the entrance site for the Acyl CoA into the active site. Surface representations of SOAT indicate that there are 2 alpha helices that block the entrance to the C tunnel, therefore a conformational change needs to occur to move the 2 helices so the substrate can enter the tunnel. The T tunnel opens into the membrane and is where cholesterol enters to have access to the active site. The two substrates are catalyzed by the H460 in the active site to form the cholesteryl ester. The products then leave via different pathways. The CoA-SH in the C tunnel leaves via that tunnel and is released back into the cytosol. The cholesteryl ester then leaves via either the T tunnel into the membrane or through the L tunnel into the lumen of the cell. <ref name="Qian" /> |
=== Active Site === | === Active Site === | ||
| Line 18: | Line 18: | ||
=== Catalytic Mechanism === | === Catalytic Mechanism === | ||
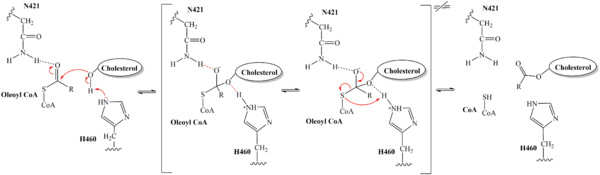
| - | The distal-most nitrogen on H460 acts as a base catalyst to deprotonate the hydroxyl group of a cholesterol molecule. This leaves the cholesterol oxygen with a negative charge, making it a good nucleophile. The [https://en.wikipedia.org/wiki/Nucleophile nucleophilic] oxygen attacks the Acyl CoA substrate at the carbonyl carbon, kicking electron density up to the carbonyl oxygen. Shown in brackets, the transition state is stabilized by N421 and newly protonated H460. [[Image:6p2pMechanism.png|600 px|right|thumb|'''Figure 5.''' | + | The distal-most nitrogen on H460 acts as a base catalyst to deprotonate the hydroxyl group of a cholesterol molecule. This leaves the cholesterol oxygen with a negative charge, making it a good nucleophile. The [https://en.wikipedia.org/wiki/Nucleophile nucleophilic] oxygen attacks the Acyl CoA substrate at the carbonyl carbon, kicking electron density up to the carbonyl oxygen. Shown in brackets, the transition state is stabilized by N421 and newly protonated H460. [[Image:6p2pMechanism.png|600 px|right|thumb|'''Figure 5.''' Mechanism for the esterification reaction of SOAT with arrow pushing.]] |
From the transition state, excess electron density on the carbonyl oxygen is collapsed back into a double bond. This causes the bond between the carbonyl carbon and sulfur to break, shifting electron density to the sulfur atom. To complete the mechanism, the negatively charged sulfur would reclaim the hydrogen from protonated H460. Acyl CoA would exit the active site as a [https://en.wikipedia.org/wiki/Leaving_group leaving group], leaving its R group attached to cholesterol in the form of a cholesterol ester. | From the transition state, excess electron density on the carbonyl oxygen is collapsed back into a double bond. This causes the bond between the carbonyl carbon and sulfur to break, shifting electron density to the sulfur atom. To complete the mechanism, the negatively charged sulfur would reclaim the hydrogen from protonated H460. Acyl CoA would exit the active site as a [https://en.wikipedia.org/wiki/Leaving_group leaving group], leaving its R group attached to cholesterol in the form of a cholesterol ester. | ||
Revision as of 21:50, 24 April 2021
Human Sterol O-acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 2.0 2.1 2.2 Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ 3.0 3.1 Bhattacharyya R, Kovacs DM. ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta. 2010 Aug;1801(8):960-5. doi: 10.1016/j.bbalip.2010.04.003. , Epub 2010 Apr 14. PMID:20398792 doi:http://dx.doi.org/10.1016/j.bbalip.2010.04.003
- ↑ 4.0 4.1 Huttunen HJ, Kovacs DM. ACAT as a drug target for Alzheimer's disease. Neurodegener Dis. 2008;5(3-4):212-4. doi: 10.1159/000113705. Epub 2008 Mar 6. PMID:18322393 doi:http://dx.doi.org/10.1159/000113705
- ↑ Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2006 Mar;38(3):151-6. doi:, 10.1111/j.1745-7270.2006.00154.x. PMID:16518538 doi:http://dx.doi.org/10.1111/j.1745-7270.2006.00154.x
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
Student Contributors
- Kylie Pfeifer
- Stephanie Pellegrino
- Kaitlyn Roberts