User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 45: | Line 45: | ||
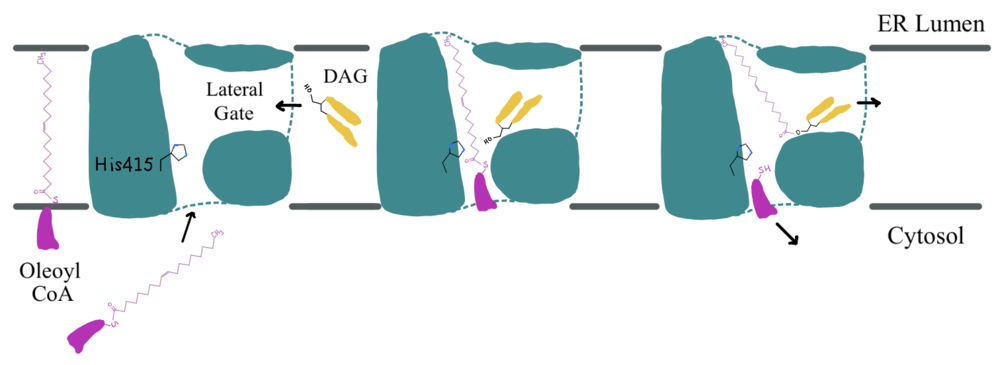
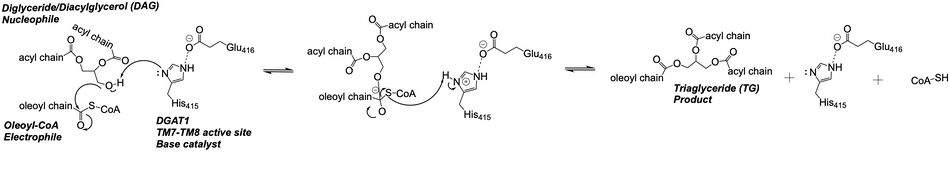
[[Image:Ch463_dgat_mech2.jpeg|950 px|center|thumb|'''Figure 3: DGAT Mechanism''' The DGAT mechanism is an acyl substitution with DAG as the nucleophile and Acyl-CoA as the electrophile. His415 is the catalytic residue that deprotonates DAG to make it a better nucleophile. Glu416 stabilizes the His415. The residue that stabilizes the oxyanion hole of the intermediate is unknown.]] | [[Image:Ch463_dgat_mech2.jpeg|950 px|center|thumb|'''Figure 3: DGAT Mechanism''' The DGAT mechanism is an acyl substitution with DAG as the nucleophile and Acyl-CoA as the electrophile. His415 is the catalytic residue that deprotonates DAG to make it a better nucleophile. Glu416 stabilizes the His415. The residue that stabilizes the oxyanion hole of the intermediate is unknown.]] | ||
| - | == Disease == | + | ==Medical Relevance== |
| + | |||
| + | === Disease === | ||
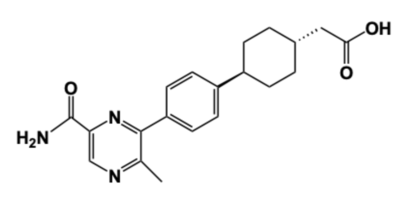
[[Image:DGAT_INHIBITOR.png|400 px|right|thumb|'''Figure 4: DGAT Inhibitor AZD7687''' Shown is the structure of AZD7687, a known inhibitor of DGAT.]] | [[Image:DGAT_INHIBITOR.png|400 px|right|thumb|'''Figure 4: DGAT Inhibitor AZD7687''' Shown is the structure of AZD7687, a known inhibitor of DGAT.]] | ||
| Line 53: | Line 55: | ||
Additionally, congenital protein-losing enteropathy ([https://www.uptodate.com/contents/protein-losing-gastroenteropathy PLE]) is linked to DGAT mutations. PLE is a GI disorder that causes malabsorption of fat and a deficiency in fat-soluble vitamins. Patients in a congenital PLE case study exhibited a homozygous missense Leu295Pro mutation within the MBOAT core of their DGAT enzymes <ref name="Stephen">PMID: 26883093</ref>. <scene name='87/877512/Mutation/4'>Leu295</scene> is located within the MBOAT core active site on TM5. While the Leu295 is not near the catalytic residues His415 and Glu416, the <scene name='87/877512/Cple/3'>Leu295Pro</scene> mutation will disrupt the overall active site. Proline is an alpha helix breaker because it causes steric hindrance within the backbone of the helix turn. It is hypothesized that this mutation breaks this helix in the MBOAT core and greatly reduces its enzymatic activity and ability to make triacylglycerides. Without proper DGAT function to produce triacylglycerides, there is a decrease in albumin, which is a protein that helps prevent fluid from leaking out of the liver and blood vessels. This decrease in albumin then leads to decreased efficiency in nutrient transport and fat absorption. | Additionally, congenital protein-losing enteropathy ([https://www.uptodate.com/contents/protein-losing-gastroenteropathy PLE]) is linked to DGAT mutations. PLE is a GI disorder that causes malabsorption of fat and a deficiency in fat-soluble vitamins. Patients in a congenital PLE case study exhibited a homozygous missense Leu295Pro mutation within the MBOAT core of their DGAT enzymes <ref name="Stephen">PMID: 26883093</ref>. <scene name='87/877512/Mutation/4'>Leu295</scene> is located within the MBOAT core active site on TM5. While the Leu295 is not near the catalytic residues His415 and Glu416, the <scene name='87/877512/Cple/3'>Leu295Pro</scene> mutation will disrupt the overall active site. Proline is an alpha helix breaker because it causes steric hindrance within the backbone of the helix turn. It is hypothesized that this mutation breaks this helix in the MBOAT core and greatly reduces its enzymatic activity and ability to make triacylglycerides. Without proper DGAT function to produce triacylglycerides, there is a decrease in albumin, which is a protein that helps prevent fluid from leaking out of the liver and blood vessels. This decrease in albumin then leads to decreased efficiency in nutrient transport and fat absorption. | ||
| - | |||
| - | == Relevance == | ||
| - | |||
| - | <ref name="Wang">PMID: 32433610</ref> | ||
| - | <ref name="Sui">PMID: 32433611</ref> | ||
| - | <ref name="Stephen">PMID: 26883093</ref> | ||
| - | <ref name="Denison">PMID: 24118885</ref> | ||
| - | <ref name="Ma">PMID: 30283133</ref> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 12:57, 26 April 2021
Diacylglycerol acyltransferase, DGAT, synthesizes triacylglycerides
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 3.2 Ma D, Wang Z, Merrikh CN, Lang KS, Lu P, Li X, Merrikh H, Rao Z, Xu W. Crystal structure of a membrane-bound O-acyltransferase. Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct, 3. PMID:30283133 doi:http://dx.doi.org/10.1038/s41586-018-0568-2
- ↑ 4.0 4.1 4.2 Denison H, Nilsson C, Lofgren L, Himmelmann A, Martensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014 Apr;16(4):334-43. doi: 10.1111/dom.12221. Epub 2013 Oct, 31. PMID:24118885 doi:http://dx.doi.org/10.1111/dom.12221
- ↑ Stephen J, Vilboux T, Haberman Y, Pri-Chen H, Pode-Shakked B, Mazaheri S, Marek-Yagel D, Barel O, Di Segni A, Eyal E, Hout-Siloni G, Lahad A, Shalem T, Rechavi G, Malicdan MC, Weiss B, Gahl WA, Anikster Y. Congenital protein losing enteropathy: an inborn error of lipid metabolism due to DGAT1 mutations. Eur J Hum Genet. 2016 Aug;24(9):1268-73. doi: 10.1038/ejhg.2016.5. Epub 2016 Feb , 17. PMID:26883093 doi:http://dx.doi.org/10.1038/ejhg.2016.5
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa