User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 45: | Line 45: | ||
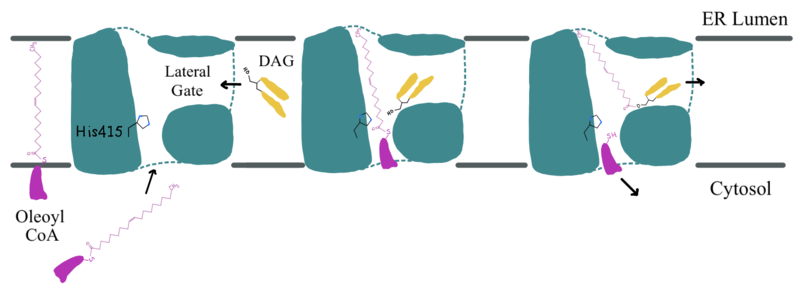
The oxyanion hole of the acyl substitution intermediate should be stabilized by DGAT. However, the two available PDB files for the substrate-bound DGAT, [https://www.rcsb.org/structure/6VZ1 6vz1] and [https://www.rcsb.org/structure/6VP0 6vp0], do not show a possible stabilizing residue for the Oleoyl-CoA, the 18 carbon chain version of Acyl-CoA. The PDB file <scene name='87/877512/Active_site_zoomed_in_6vz1/2'>6vz1</scene> shows the sulfur of Oleoyl-CoA, the electrophile of the mechanism, facing away from His415. This is not likely to be the proper orientation for Oleoyl-CoA, as this sulfur would have to be facing towards the His415 to be protonated after the acyl substitution. For an oxyanion stabilizing residue to be located within this PDB file, the Oleyl-CoA would have to be properly oriented. The other substrate-bound PDB file, <scene name='87/877512/Active_site_zoomed_in_6vp0/1'>6vp0</scene>, shows the sulfur of Oleyl-CoA facing the His415. However, the tail of Oleyl-CoA is curved around the oxyanion hole, preventing a stabilizing residue within DGAT to be found. For these reasons, an oxyanion stabilizing residue was not identified in the mechanism nor in the images. | The oxyanion hole of the acyl substitution intermediate should be stabilized by DGAT. However, the two available PDB files for the substrate-bound DGAT, [https://www.rcsb.org/structure/6VZ1 6vz1] and [https://www.rcsb.org/structure/6VP0 6vp0], do not show a possible stabilizing residue for the Oleoyl-CoA, the 18 carbon chain version of Acyl-CoA. The PDB file <scene name='87/877512/Active_site_zoomed_in_6vz1/2'>6vz1</scene> shows the sulfur of Oleoyl-CoA, the electrophile of the mechanism, facing away from His415. This is not likely to be the proper orientation for Oleoyl-CoA, as this sulfur would have to be facing towards the His415 to be protonated after the acyl substitution. For an oxyanion stabilizing residue to be located within this PDB file, the Oleyl-CoA would have to be properly oriented. The other substrate-bound PDB file, <scene name='87/877512/Active_site_zoomed_in_6vp0/1'>6vp0</scene>, shows the sulfur of Oleyl-CoA facing the His415. However, the tail of Oleyl-CoA is curved around the oxyanion hole, preventing a stabilizing residue within DGAT to be found. For these reasons, an oxyanion stabilizing residue was not identified in the mechanism nor in the images. | ||
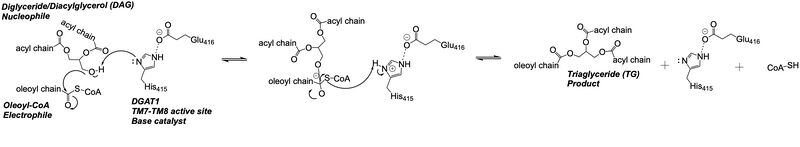
| - | [[Image:Ch463_dgat_mech2.jpeg| | + | [[Image:Ch463_dgat_mech2.jpeg|800 px|center|thumb|'''Figure 3: DGAT Mechanism''' The DGAT mechanism is an acyl substitution with DAG as the nucleophile and Acyl-CoA as the electrophile. His415 is the catalytic residue that deprotonates DAG to make it a better nucleophile. Glu416 stabilizes the His415. The residue that stabilizes the oxyanion hole of the intermediate is unknown.]] |
==Medical Relevance== | ==Medical Relevance== | ||
Revision as of 19:15, 26 April 2021
Diacylglycerol acyltransferase, DGAT, synthesizes triacylglycerides
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 3.2 Ma D, Wang Z, Merrikh CN, Lang KS, Lu P, Li X, Merrikh H, Rao Z, Xu W. Crystal structure of a membrane-bound O-acyltransferase. Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct, 3. PMID:30283133 doi:http://dx.doi.org/10.1038/s41586-018-0568-2
- ↑ 4.0 4.1 4.2 Denison H, Nilsson C, Lofgren L, Himmelmann A, Martensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014 Apr;16(4):334-43. doi: 10.1111/dom.12221. Epub 2013 Oct, 31. PMID:24118885 doi:http://dx.doi.org/10.1111/dom.12221
- ↑ Stephen J, Vilboux T, Haberman Y, Pri-Chen H, Pode-Shakked B, Mazaheri S, Marek-Yagel D, Barel O, Di Segni A, Eyal E, Hout-Siloni G, Lahad A, Shalem T, Rechavi G, Malicdan MC, Weiss B, Gahl WA, Anikster Y. Congenital protein losing enteropathy: an inborn error of lipid metabolism due to DGAT1 mutations. Eur J Hum Genet. 2016 Aug;24(9):1268-73. doi: 10.1038/ejhg.2016.5. Epub 2016 Feb , 17. PMID:26883093 doi:http://dx.doi.org/10.1038/ejhg.2016.5
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa