We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Fleshman/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | =Acyl-Coenzyme Cholesterol Acetyltransferase ( | + | =Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1): Function, Structure, and Inhibition= |
<StructureSection load='6p2p' size='340' side='right' caption='ACAT' scene='87/877605/6p2p_dimer/6'> | <StructureSection load='6p2p' size='340' side='right' caption='ACAT' scene='87/877605/6p2p_dimer/6'> | ||
==Introduction== | ==Introduction== | ||
| - | Acyl-Coenzyme A Cholesterol Acyltransferase ( | + | Acyl-Coenzyme: A Cholesterol Acyltransferase 1 (ACAT1), or also known as Sterol ''O''-Acyltransferase (SOAT), is an important enzyme in the body. |
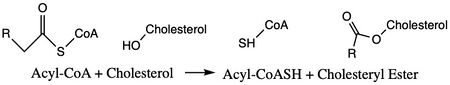
| - | Cholesterol esters were found in arterial lesions in 1910, but the first | + | [https://en.wikipedia.org/wiki/Sterol_O-acyltransferase ACAT1] is an important enzyme that catalyzes the esterification of cholesterol to form cholesterol esters, and it belongs to the class of enzymes called [https://en.wikipedia.org/wiki/Acyltransferase acyltransferases] (Figure 1). As a member of the [https://en.wikipedia.org/wiki/MBOAT MBOAT] family, it is a key enzyme in lipid metabolism. This enzyme is biologically important because it affects the solubility of cholesterol in the cell membrane and promotes accumulation of cholesterol ester in the cytoplasm as fat droplets. Accumulation of cholesterol ester as these lipid droplets is a main characteristic of |
| + | [https://en.wikipedia.org/wiki/Foam_cell macrophage foaming], which can lead to | ||
| + | [https://en.wikipedia.org/wiki/Atherosclerosis atherosclerotic diseases] <ref name=”Qian”>PMID:32433614</ref>. | ||
| + | Cholesterol esters were found in arterial lesions in 1910, but the first ACAT1 activity was discovered in the mid 1900's. This led to the inhibition of ACAT1 as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT1 inhibitors grew, but some of the compounds developed exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT1 gene was successfully cloned. This discovery led to more studies with ACAT1 and atherosclerosis. Some of these studies used mice and also showed cellular toxicity. ACAT1 inhibition is still being pursued as a strategy for treatment or prevention of atherosclerosis and related diseases. | ||
<ref name=”Farese Jr.”>PMID: 16857957</ref> | <ref name=”Farese Jr.”>PMID: 16857957</ref> | ||
| - | [[Image:simplemechanismforACAT.jpg|450 px|left|thumb|Figure 1. | + | [[Image:simplemechanismforACAT.jpg|450 px|left|thumb|Figure 1. Chemical Structures for the Reactants and Products of ACAT1]] |
| - | + | ||
==Structural Overview== | ==Structural Overview== | ||
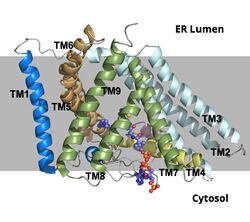
| - | + | ACAT1 was experimentally determined as a <scene name='87/877604/Tetramer/3'>tetramer</scene> of size approximately 260 kDa, composed of helices and loops <ref name=”Shengcheng”>PMID:32424158</ref>. Each monomer of the [https://en.wikipedia.org/wiki/Tetramer tetramer] contains 9 transmembrane helices (Figure 3) <ref name=”Qian”>PMID:32433614</ref>. However, the <scene name='87/877604/Colored_dimer/5'>dimer of ACAT1</scene> was found to be the biologically active arrangement. | |
| - | + | [[Image:Screen_Shot_2021-04-20_at_3.29.54_PM.jpg|400 px|left|thumb|Figure 2. ACAT1 Dimer in the Membrane. The gray shaded region is the plane of the bilipid membrane. The tunnels where molecules enter and exit are labeled. The ER lumen is at the top with the cytosol at the bottom.]] | |
| - | [[Image:Screen_Shot_2021-04-20_at_3.29.54_PM.jpg|400 px|left|thumb|Figure 2. The | + | |
| - | The <scene name='87/877604/Dimer_interface/ | + | ===Dimer-Dimer Interface=== |
| - | + | The <scene name='87/877604/Dimer_interface/18'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner <ref name=”Shengcheng”>PMID:32424158</ref>. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels necessary for substrates to enter them. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway <ref name=”Qian”>PMID:32433614</ref>. [[Image:Screen_Shot_2021-04-20_at_3.30.39_PM.jpg|250 px|right|thumb|Figure 3. ACAT1 Monomer in the Membrane. This shows the 9 transmembrane helices. Each helix is labeled and colored according to the different domains. The ligand is shown as ball and stick.]] | |
| - | [[Image:Screen_Shot_2021-04-20_at_3.30.39_PM.jpg|250 px|right|thumb|Figure 3. Monomer in the Membrane. This shows the 9 transmembrane helices. Each helix is labeled and colored according to the different domains. The ligand is shown as ball and stick.]] | + | |
===Tunnels=== | ===Tunnels=== | ||
| Line 25: | Line 25: | ||
| - | ==Active Site== | + | ===Active Site=== |
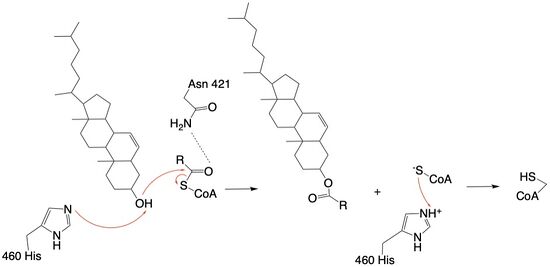
The catalytic site contains <scene name='87/877605/Catalytic_residues/2'>catalytic residues</scene> that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid (Trp407,Trp420). The active site is at the intersection of all three tunnels to allow a central position for the acyltransferase to occur. | The catalytic site contains <scene name='87/877605/Catalytic_residues/2'>catalytic residues</scene> that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid (Trp407,Trp420). The active site is at the intersection of all three tunnels to allow a central position for the acyltransferase to occur. | ||
| Line 46: | Line 46: | ||
</StructureSection> | </StructureSection> | ||
| - | == References == | + | ==References== |
| - | + | ||
| - | + | ||
<references/> | <references/> | ||
==Student Contributors== | ==Student Contributors== | ||
*Megan Fleshman, Tori Templin, Haylie Moehlenkamp | *Megan Fleshman, Tori Templin, Haylie Moehlenkamp | ||
Revision as of 21:23, 26 April 2021
Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1): Function, Structure, and Inhibition
| |||||||||||
References
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ 10.0 10.1 10.2 10.3 10.4 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 11.0 11.1 11.2 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
- ↑ Vaziri ND, Liang KH. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004 Jul 27;110(4):419-25. doi: 10.1161/01.CIR.0000136023.70841.0F. , Epub 2004 Jul 19. PMID:15262831 doi:http://dx.doi.org/10.1161/01.CIR.0000136023.70841.0F
- ↑ Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1262-7. doi: 10.1073/pnas.0336398100., Epub 2003 Jan 21. PMID:12538880 doi:http://dx.doi.org/10.1073/pnas.0336398100
- ↑ 15.0 15.1 Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. PMID:26669800 doi:http://dx.doi.org/10.4155/fmc.15.161
Student Contributors
- Megan Fleshman, Tori Templin, Haylie Moehlenkamp