We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 39: | Line 39: | ||
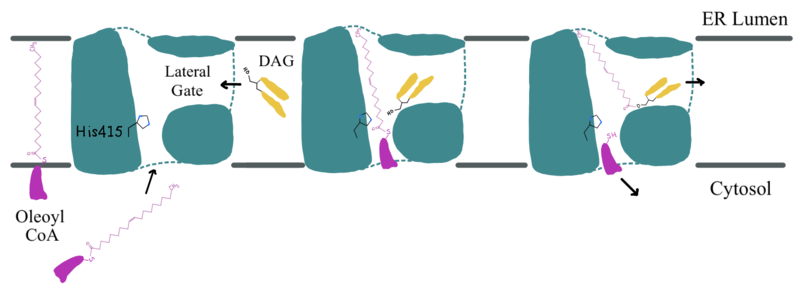
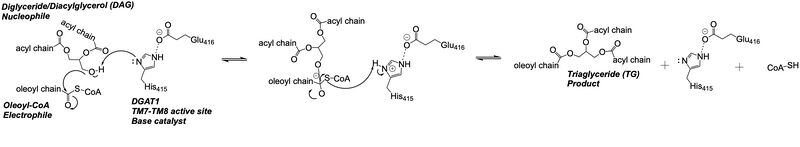
The <scene name='87/877512/Active_site_zoomed_out/4'>active site</scene> is located within the pocket formed from TM2-TM9, IL1, and IL2, with the catalytic residue Histidine 415 located on TM7. It is accessible through openings both on the cytosolic and luminal sides. The active site of DGAT1 serves its catalytic function by placing the His415 residue in close proximity to the acyl-CoA in order to cleave its ester bond and bind the fatty acid to the diacylglycerol. The conserved His415 acts catalytically by a [https://www.genscript.com/molecular-biology-glossary/478/charge-relay-system#:~:text=A%20tautomeric%20form%20of%20a,another%20in%20the%20same%20protein charge relay system], where the negative charge of the neighboring Glu416 pulls on the electrons of histidine at the N1 position, making the N3 position more nucleophilic. This nitrogen will then deprotonate DAG so it can begin its attack on Acyl-CoA through [https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/21%3A_Carboxylic_Acid_Derivatives-_Nucleophilic_Acyl_Substitution_Reactions/21.02%3A_Nucleophilic_Acyl_Substitution_Reactions acyl substitution]. The catalytic mechanism for DGAT1 is shown in Figure 3 <ref name="Wang">PMID: 32433610</ref>. | The <scene name='87/877512/Active_site_zoomed_out/4'>active site</scene> is located within the pocket formed from TM2-TM9, IL1, and IL2, with the catalytic residue Histidine 415 located on TM7. It is accessible through openings both on the cytosolic and luminal sides. The active site of DGAT1 serves its catalytic function by placing the His415 residue in close proximity to the acyl-CoA in order to cleave its ester bond and bind the fatty acid to the diacylglycerol. The conserved His415 acts catalytically by a [https://www.genscript.com/molecular-biology-glossary/478/charge-relay-system#:~:text=A%20tautomeric%20form%20of%20a,another%20in%20the%20same%20protein charge relay system], where the negative charge of the neighboring Glu416 pulls on the electrons of histidine at the N1 position, making the N3 position more nucleophilic. This nitrogen will then deprotonate DAG so it can begin its attack on Acyl-CoA through [https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/21%3A_Carboxylic_Acid_Derivatives-_Nucleophilic_Acyl_Substitution_Reactions/21.02%3A_Nucleophilic_Acyl_Substitution_Reactions acyl substitution]. The catalytic mechanism for DGAT1 is shown in Figure 3 <ref name="Wang">PMID: 32433610</ref>. | ||
| - | The oxyanion hole of the acyl substitution intermediate should be stabilized by DGAT1. However, the two available PDB files for the substrate-bound DGAT1, [https://www.rcsb.org/structure/6VZ1 6vz1] and [https://www.rcsb.org/structure/6VP0 6vp0], do not show a possible stabilizing residue for the Oleoyl-CoA, the 18 carbon chain version of Acyl-CoA. The PDB file 6vz1 shows the sulfur of Oleoyl-CoA, the electrophile of the mechanism, <scene name='87/877512/Active_site_zoomed_in_6vz1/3'>facing away from His415</scene>. This is not likely to be the proper orientation for Oleoyl-CoA, as this sulfur would have to be facing towards the His415 to be protonated after the acyl substitution. For an oxyanion stabilizing residue to be located within this PDB file, the Oleoyl-CoA would have to be properly oriented. The other substrate-bound PDB file 6vp0 shows the sulfur of Oleoyl-CoA facing toward the His415. However, <scene name=' | + | The oxyanion hole of the acyl substitution intermediate should be stabilized by DGAT1. However, the two available PDB files for the substrate-bound DGAT1, [https://www.rcsb.org/structure/6VZ1 6vz1] and [https://www.rcsb.org/structure/6VP0 6vp0], do not show a possible stabilizing residue for the Oleoyl-CoA, the 18 carbon chain version of Acyl-CoA. The PDB file 6vz1 shows the sulfur of Oleoyl-CoA, the electrophile of the mechanism, <scene name='87/877512/Active_site_zoomed_in_6vz1/3'>facing away from His415</scene>. This is not likely to be the proper orientation for Oleoyl-CoA, as this sulfur would have to be facing towards the His415 to be protonated after the acyl substitution. For an oxyanion stabilizing residue to be located within this PDB file, the Oleoyl-CoA would have to be properly oriented. The other substrate-bound PDB file 6vp0 shows the sulfur of Oleoyl-CoA facing toward the His415. However, <scene name='87/877512/Active_site_zoomed_in_6vp0/2'>the tail of oleoyl-CoA is bent</scene> and curved around the oxyanion hole, preventing a stabilizing residue within DGAT1 to be found. For these reasons, an oxyanion stabilizing residue was not identified in the mechanism nor in the images. |
[[Image:Ch463_dgat_mech2.jpeg|800 px|center|thumb|'''Figure 3: DGAT1 Mechanism''' The DGAT1 mechanism is an acyl substitution with DAG as the nucleophile and Acyl-CoA as the electrophile. His415 is the catalytic residue that deprotonates DAG to make it a better nucleophile. Glu416 stabilizes the His415. The residue that stabilizes the oxyanion hole of the intermediate is unknown.]] | [[Image:Ch463_dgat_mech2.jpeg|800 px|center|thumb|'''Figure 3: DGAT1 Mechanism''' The DGAT1 mechanism is an acyl substitution with DAG as the nucleophile and Acyl-CoA as the electrophile. His415 is the catalytic residue that deprotonates DAG to make it a better nucleophile. Glu416 stabilizes the His415. The residue that stabilizes the oxyanion hole of the intermediate is unknown.]] | ||
Revision as of 22:33, 26 April 2021
Diacylglycerol acyltransferase 1, DGAT1, synthesizes triacylglycerides
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 3.2 Ma D, Wang Z, Merrikh CN, Lang KS, Lu P, Li X, Merrikh H, Rao Z, Xu W. Crystal structure of a membrane-bound O-acyltransferase. Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct, 3. PMID:30283133 doi:http://dx.doi.org/10.1038/s41586-018-0568-2
- ↑ 4.0 4.1 4.2 4.3 Denison H, Nilsson C, Lofgren L, Himmelmann A, Martensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014 Apr;16(4):334-43. doi: 10.1111/dom.12221. Epub 2013 Oct, 31. PMID:24118885 doi:http://dx.doi.org/10.1111/dom.12221
- ↑ Stephen J, Vilboux T, Haberman Y, Pri-Chen H, Pode-Shakked B, Mazaheri S, Marek-Yagel D, Barel O, Di Segni A, Eyal E, Hout-Siloni G, Lahad A, Shalem T, Rechavi G, Malicdan MC, Weiss B, Gahl WA, Anikster Y. Congenital protein losing enteropathy: an inborn error of lipid metabolism due to DGAT1 mutations. Eur J Hum Genet. 2016 Aug;24(9):1268-73. doi: 10.1038/ejhg.2016.5. Epub 2016 Feb , 17. PMID:26883093 doi:http://dx.doi.org/10.1038/ejhg.2016.5
- ↑ Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, Farese RV Jr. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009 Aug;50(2):434-42. doi: 10.1002/hep.22980. PMID:19472314 doi:http://dx.doi.org/10.1002/hep.22980
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa