User:Hannah Wright/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
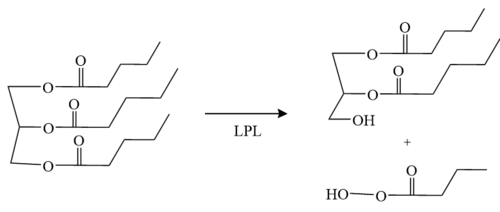
'''Figure 1:''' Conversion of triglyceride to diglyceride by LPL. | '''Figure 1:''' Conversion of triglyceride to diglyceride by LPL. | ||
| - | LPL was identified more than 60 years ago and studied by biochemists and physiologists intensely since. It wasn’t until recently that LPL’s detailed structure was determined due to LPL’s hydrolase domain susceptibility to unfolding. LMF1 and GPIHBP1, '''glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1''' ,were discovered to be required for proper folding and enzymatic activity of LPL. LMF1,''' lipase maturation factor 1''', is a chaperone protein that is responsible for proper folding and secretion of LPL. Through the use of X-ray crystallography it was also discovered that LPL is a monomer rather than the previously believed homodimer. | + | LPL was identified more than 60 years ago and studied by biochemists and physiologists intensely since. It wasn’t until recently that LPL’s detailed structure was determined due to LPL’s hydrolase domain susceptibility to unfolding. LMF1 and GPIHBP1, '''glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1''' , were discovered to be required for proper folding and enzymatic activity of LPL. LMF1,''' lipase maturation factor 1''', is a chaperone protein that is responsible for proper folding and secretion of LPL. Through the use of X-ray crystallography, it was also discovered that LPL is a monomer rather than the previously believed homodimer. |
</div> | </div> | ||
| Line 15: | Line 15: | ||
LPL is located within the interstitial space independently and remains stranded there if not acted upon by GPIHBP1. GPIHBP1 then captures LPL in the interstitial spaces and shuttles it across endothelial cells into the capillary lumen. Once in the capillaries, the LPL-GPIHBP1 complex catalyzes the breakdown of triglycerides in the blood. In doing so, it prevents high levels of triglycerides in the plasma to provide nutrients for vital tissues. | LPL is located within the interstitial space independently and remains stranded there if not acted upon by GPIHBP1. GPIHBP1 then captures LPL in the interstitial spaces and shuttles it across endothelial cells into the capillary lumen. Once in the capillaries, the LPL-GPIHBP1 complex catalyzes the breakdown of triglycerides in the blood. In doing so, it prevents high levels of triglycerides in the plasma to provide nutrients for vital tissues. | ||
===Significance=== | ===Significance=== | ||
| - | Lipoprotein lipase deficiency leads to [ | + | Lipoprotein lipase deficiency leads to [http://.en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia] (elevated levels of triglycerides in the blood) also known as chylomicronemia. This can go on to increase insulin resistance and the risk of obesity. |
== Structure == | == Structure == | ||
| Line 32: | Line 32: | ||
GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the hydrophobic effect and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL. | GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the hydrophobic effect and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL. | ||
====Calcium Ion Coordination==== | ====Calcium Ion Coordination==== | ||
| - | The calcium ion has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the side chain of D201, for example, can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain. | + | The calcium ion has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the side chain of D201, for example, can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. |
===Active Site=== | ===Active Site=== | ||
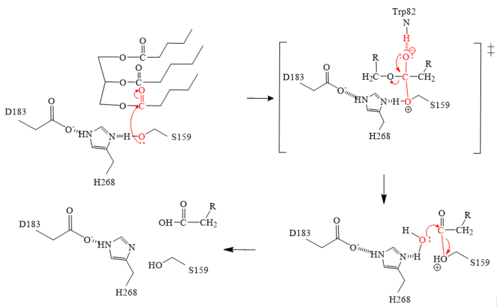
| - | The <scene name='87/878236/Active_site_4_20/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_fixed/9'>hydrophobic entry</scene> to the binding site outlines the general structure and provides considerable stability to the active site. The <scene name='87/878236/Active_site_lid_region_fixed/5'>lid region</scene> is also an important component of the active site, occupying residues 243-266, which is vital for the recognition of substrates. The <scene name='87/878236/Active_site_w_catalytic_residu/6'>catalytic residues</scene> are H268, S159, and D183 which catalyze the reaction of the typical substrate of LPL, triglycerides. The oxyanion hole, consisting of residues L160, and W82, aids in the overall stability of the active site and the transition state of substrates. The main chain nitrogens stabilize the tetrahedral intermediate <ref name="Birrane">PMID:30559189</ref>. | + | The <scene name='87/878236/Active_site_4_20/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_fixed/9'>hydrophobic entry</scene> to the binding site outlines the general structure and provides considerable stability to the active site. The <scene name='87/878236/Active_site_lid_region_fixed/5'>lid region</scene> is also an important component of the active site, occupying residues 243-266, which is vital for the recognition of substrates. The <scene name='87/878236/Active_site_w_catalytic_residu/6'>catalytic residues</scene> are H268, S159, and D183 (also known as the [http://en.wikipedia.org/wiki/Catalytic_triad#:~:text=A%20catalytic%20triad%20is%20a,lipases%20and%20%CE%B2%2Dlactamases) catalytic triad] which catalyze the reaction of the typical substrate of LPL, triglycerides. The oxyanion hole, consisting of residues L160, and W82, aids in the overall stability of the active site and the transition state of substrates. The main chain nitrogens stabilize the tetrahedral intermediate <ref name="Birrane">PMID:30559189</ref>. |
====Mechanism==== | ====Mechanism==== | ||
| Line 42: | Line 42: | ||
# The triglyceride binds to LPL’s lipid-binding region in an open lid conformation. | # The triglyceride binds to LPL’s lipid-binding region in an open lid conformation. | ||
| - | # The oxygen on S159 is made more [ | + | # The oxygen on S159 is made more [http://en.wikipedia.org/wiki/Nucleophile nucleophilic]. This happens via [http://en.wikipedia.org/wiki/Histidine histidine] hydrogen bonding with the hydrogen on S159’s alcohol group. |
| - | # The nucleophilic oxygen attacks the [ | + | # The nucleophilic oxygen attacks the [http://en.wikipedia.org/wiki/Carbonyl_group carbonyl carbon] of one of the fatty acid chains. |
# This pushes electrons up onto the carbonyl oxygen, creating a [http://www.chem.ucla.edu/~harding/IGOC/T/tetrahedral_intermediate.html tetrahedral intermediate]. This is the oxyanion hole which is stabilized by main chain nitrogen atoms of W82 and L160. | # This pushes electrons up onto the carbonyl oxygen, creating a [http://www.chem.ucla.edu/~harding/IGOC/T/tetrahedral_intermediate.html tetrahedral intermediate]. This is the oxyanion hole which is stabilized by main chain nitrogen atoms of W82 and L160. | ||
| - | # One of the lone pairs | + | # One of the lone pairs on the oxygen (in the oxyanion hole) creates a double bond carbon. |
| - | # The oxygen-carbon bond between the single fatty acid chain and the [ | + | # The oxygen-carbon bond between the single fatty acid chain and the [http://en.wikipedia.org/wiki/Diglyceride diglyceride] is cleaved. |
# H268 hydrogen bonds water, making the oxygen a better nucleophile. Water attacks the carbonyl carbon. | # H268 hydrogen bonds water, making the oxygen a better nucleophile. Water attacks the carbonyl carbon. | ||
| - | # The [ | + | # The [http://en.wikipedia.org/wiki/Carboxylic_acid carboxylic acid] is formed and the S159 bond is cleaved and re-protonated via H268. |
# The active site is now back in its original state. | # The active site is now back in its original state. | ||
| Line 56: | Line 56: | ||
===Mutations=== | ===Mutations=== | ||
====D201V==== | ====D201V==== | ||
| - | <scene name='87/877636/D201_mutation/11'>D201V</scene> is a mutation that is found to cause [ | + | <scene name='87/877636/D201_mutation/11'>D201V</scene> is a mutation that is found to cause [http://en.wikipedia.org/wiki/Lipoprotein_lipase_deficiency chylomicronemia]. Chylomicronemia is when the body cannot break down lipids properly. This leads to their build-up in the body causing high levels of triglycerides in the body. The [http://en.wikipedia.org/wiki/Aspartic_acid carboxyl side chain of aspartate] 201 is one of the coordination sites for the calcium ion of LPL. The mutation to hydrophobic [http://en.wikipedia.org/wiki/Valine valine] means the loss of this coordination site<ref name="Birrane">PMID:30559189</ref>. This mutation adversely affects the folding of LPL and thus affects the secretion of LPL, overall decreasing the activity of LPL<ref name="Birrane">PMID:30559189</ref>. |
====M404R==== | ====M404R==== | ||
| - | <scene name='87/877636/M404r_1/3'>M404R</scene> is a mutation found within LPL that caused [ | + | <scene name='87/877636/M404r_1/3'>M404R</scene> is a mutation found within LPL that caused [http://en.wikipedia.org/wiki/Lipoprotein_lipase_deficiency chylomicronemia] in patients. The hydrophobic [http://en.wikipedia.org/wiki/Methionine methionine] is mutated to the larger and charged side chain of [http://en.wikipedia.org/wiki/Arginine arginine]. Originally it was thought to impact LPL secretion from cells. It was found that the M404R does not affect LPL secretion <ref name="Birrane">PMID:30559189</ref>. M404R interacts with the hydrophobic pocket of GPIHBP1’s finger 3 of its 3 fingered domain (V121, E122, T124, V126). The large, charged arginine repelled the hydrophobic pocket and does not fit well. This prevents proper binding and formation of the LPL-GPIHBP1 complex <ref name="Birrane">PMID:30559189</ref>. |
| Line 68: | Line 68: | ||
==Student/Contributors== | ==Student/Contributors== | ||
| - | *Ashrey | + | *Ashrey Burley |
*Allison Welz | *Allison Welz | ||
*Hannah Wright | *Hannah Wright | ||
Revision as of 23:07, 26 April 2021
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
Student/Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright