User:Hannah Wright/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
The calcium ion has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the side chain of D201, for example, can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. | The calcium ion has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the side chain of D201, for example, can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. | ||
===Active Site=== | ===Active Site=== | ||
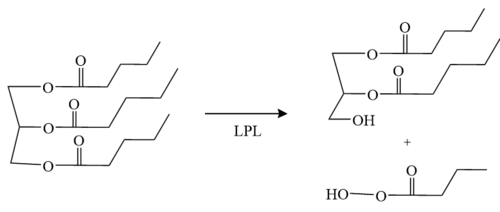
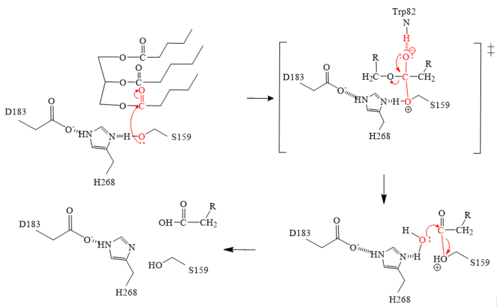
| - | The <scene name='87/878236/Active_site_4_20/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_fixed/9'>hydrophobic entry</scene> to the binding site outlines the general structure and provides considerable stability to the active site. The <scene name='87/878236/Active_site_lid_region_fixed/5'>lid region</scene> is also an important component of the active site, occupying residues 243-266, which is vital for the recognition of substrates. The <scene name='87/878236/Active_site_w_catalytic_residu/6'>catalytic residues</scene> are H268, S159, and D183 (also known as the [http://en.wikipedia.org/wiki/Catalytic_triad#:~:text=A%20catalytic%20triad%20is%20a,lipases%20and%20%CE%B2%2Dlactamases) catalytic triad] which catalyze the reaction of the typical substrate of LPL, triglycerides. The oxyanion hole, consisting of residues L160, and W82, aids in the | + | The <scene name='87/878236/Active_site_4_20/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_fixed/9'>hydrophobic entry</scene> to the binding site outlines the general structure and provides considerable stability to the active site. The <scene name='87/878236/Active_site_lid_region_fixed/5'>lid region</scene> is also an important component of the active site, occupying residues 243-266, which is vital for the recognition of substrates. The <scene name='87/878236/Active_site_w_catalytic_residu/6'>catalytic residues</scene> are H268, S159, and D183 (also known as the [http://en.wikipedia.org/wiki/Catalytic_triad#:~:text=A%20catalytic%20triad%20is%20a,lipases%20and%20%CE%B2%2Dlactamases) catalytic triad] which catalyze the reaction of the typical substrate of LPL, triglycerides. The [http://en.wikipedia.org/wiki/Oxyanion_hole#:~:text=An%20oxyanion%20hole%20is%20a,amides%20or%20positively%20charged%20residues oxyanion hole], consisting of residues L160, and W82, aids in the stability of the transition state of substrates. The main chain nitrogens stabilize the tetrahedral intermediate <ref name="Birrane">PMID:30559189</ref>. |
====Mechanism==== | ====Mechanism==== | ||
Revision as of 23:09, 26 April 2021
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
Student/Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright