User:Brianna Avery/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
====Binding Pocket==== | ====Binding Pocket==== | ||
SCD1 consists of a hydrophobic <scene name='87/877504/Baseline_structure/7'>V-shaped tunnel</scene> deep within the protein that the substrates will enter <ref name="Larochelle">DOI: 10.1038/nsmb.3049</ref>. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals. Precise placement of the <scene name='87/877504/Zn_h_bond_stabilization_2/9'>C9-C10</scene> atoms near the two iron metals provides the tunnel with regioselectivity and stereospecificity, stabilizing the substrate to allow for desaturation at carbons 9 and 10. The kink is formed by two conserved <scene name='87/877504/Trp_thr_creation_of_kink/4'>Trp149 and Thr257 residues</scene> <ref name="Bai" />. It is at this kink of the tunnel where desaturation occurs. | SCD1 consists of a hydrophobic <scene name='87/877504/Baseline_structure/7'>V-shaped tunnel</scene> deep within the protein that the substrates will enter <ref name="Larochelle">DOI: 10.1038/nsmb.3049</ref>. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals. Precise placement of the <scene name='87/877504/Zn_h_bond_stabilization_2/9'>C9-C10</scene> atoms near the two iron metals provides the tunnel with regioselectivity and stereospecificity, stabilizing the substrate to allow for desaturation at carbons 9 and 10. The kink is formed by two conserved <scene name='87/877504/Trp_thr_creation_of_kink/4'>Trp149 and Thr257 residues</scene> <ref name="Bai" />. It is at this kink of the tunnel where desaturation occurs. | ||
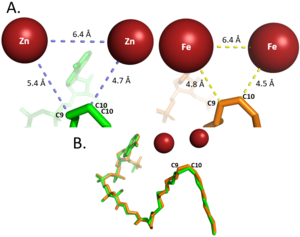
| - | + | [[Image:Use_Metal_Cation_Figure.png|300 px|right|thumb|'''Figure 2: Differences between zinc and iron di-metal interactions with acyl-CoA.''']] | |
====Metal Cations==== | ====Metal Cations==== | ||
| - | + | Typically, SCD1 contains a <scene name='87/877504/Di_metal_center/3'>di-metal center</scene> in the core of the protein that contributes to its desaturation function. Crystallized structures of SCD1 indicated a substitution of zinc as the di-metal center instead of iron<ref name="Bai" />. It is proposed that the difference in size of Zinc and Iron (8.8 Å and 9.2 Å respectively) would not affect the structure of SCD. However, the substitution of Zinc as the di-iron center is found to inhibit the function of SCD1 due to a farther distance between the two metals compared to iron (Figure containing all 3 of Treys photos) <ref name="Bai" />. | |
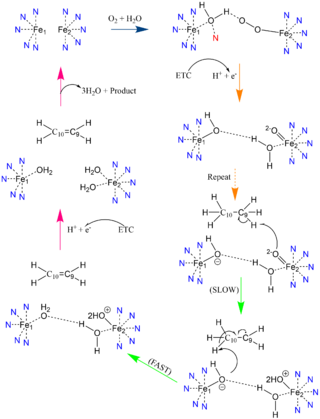
In other di-metal centered enzymes such as [https://en.wikipedia.org/wiki/Acyl-(acyl-carrier-protein)_desaturase ACP desaturase] and [https://en.wikipedia.org/wiki/Ribonucleotide_reductase ribonucleotide reductase], the enzymatic mechanism involves an oxo-bridge: a water molecule that is recruited by the di-iron center to be directly involved in the desaturase mechanism. This water gets deprotonated by the two metal ions and it becomes nucleophilic enough to attack the substrate (Mechanism figure). Based on electron density mapping of SCD1, this oxo-bridge formation is suggested to be short-lived <ref name="Shen">DOI: 10.1016/j.jmb.2020.05.017</ref>. | In other di-metal centered enzymes such as [https://en.wikipedia.org/wiki/Acyl-(acyl-carrier-protein)_desaturase ACP desaturase] and [https://en.wikipedia.org/wiki/Ribonucleotide_reductase ribonucleotide reductase], the enzymatic mechanism involves an oxo-bridge: a water molecule that is recruited by the di-iron center to be directly involved in the desaturase mechanism. This water gets deprotonated by the two metal ions and it becomes nucleophilic enough to attack the substrate (Mechanism figure). Based on electron density mapping of SCD1, this oxo-bridge formation is suggested to be short-lived <ref name="Shen">DOI: 10.1016/j.jmb.2020.05.017</ref>. | ||
| Line 44: | Line 44: | ||
==Related Disease== | ==Related Disease== | ||
| - | ===Diabetes and Obesity=== | + | ====Diabetes and Obesity==== |
Metabolic-related issues such as obesity and diabetes are linked to having a diet high in saturated fats and simple sugars <ref name="Invest">PMID: 16741579</ref>. SCD1 catalyzes the desaturation of fully saturated fatty to monounsaturated fatty acid chains and acts as the rate limiting step for this type of desaturation mechanism <ref name="Yokoyama">PMID: 22326531</ref>. The deficiency and/or inhibition of SCD1 results in increased insulin sensitivity, reduced body obesity and resistance to diet-induced obesity. | Metabolic-related issues such as obesity and diabetes are linked to having a diet high in saturated fats and simple sugars <ref name="Invest">PMID: 16741579</ref>. SCD1 catalyzes the desaturation of fully saturated fatty to monounsaturated fatty acid chains and acts as the rate limiting step for this type of desaturation mechanism <ref name="Yokoyama">PMID: 22326531</ref>. The deficiency and/or inhibition of SCD1 results in increased insulin sensitivity, reduced body obesity and resistance to diet-induced obesity. | ||
In the medical field, SCD1 is being used as a target for treatment of obesity, diabetes, and other metabolic diseases <ref name="Bai" />. In previous research, a decrease in SCD1 has shown to decrease the expression of lipogenic genes such as [https://proteopedia.org/wiki/index.php/Fatty_acid_synthase fatty acid synthase] (FAS), [https://proteopedia.org/wiki/index.php/Acetyl-CoA_carboxylase acetyl CoA carboxylase](ACC), and [https://en.wikipedia.org/wiki/Cholesterol_7_alpha-hydroxylase cholesterol 7 alpha-hydroxylase] (CYP7A 1) <ref name="Invest" />. SCD1-deficiency causing lowered expression of these fat-synthesizing genes also represses glycolysis and gluconeogenesis. SCD1 deficiency shows an increase in insulin sensitivity and increase resistance to diet-influenced obesity <ref name="Ntambi">PMID: 12177411</ref>. Changes in cellular concentration of this enzyme can cause positive or negative affects to metabolic processes that are linked with diabetes and obesity. | In the medical field, SCD1 is being used as a target for treatment of obesity, diabetes, and other metabolic diseases <ref name="Bai" />. In previous research, a decrease in SCD1 has shown to decrease the expression of lipogenic genes such as [https://proteopedia.org/wiki/index.php/Fatty_acid_synthase fatty acid synthase] (FAS), [https://proteopedia.org/wiki/index.php/Acetyl-CoA_carboxylase acetyl CoA carboxylase](ACC), and [https://en.wikipedia.org/wiki/Cholesterol_7_alpha-hydroxylase cholesterol 7 alpha-hydroxylase] (CYP7A 1) <ref name="Invest" />. SCD1-deficiency causing lowered expression of these fat-synthesizing genes also represses glycolysis and gluconeogenesis. SCD1 deficiency shows an increase in insulin sensitivity and increase resistance to diet-influenced obesity <ref name="Ntambi">PMID: 12177411</ref>. Changes in cellular concentration of this enzyme can cause positive or negative affects to metabolic processes that are linked with diabetes and obesity. | ||
| - | ===Cancer=== | + | ====Cancer==== |
SCD1 works to keep a balance between saturated and monosaturated fatty acids, and in turn, this helps prevent the build up of toxic saturated fats inside the cell<ref name="Holder" />. Increased expression levels of SCD1 has shown to enhance cancer cell proliferation. The concentration of monounsaturated fatty acids (MUFAs) is much higher in a cancer cell versus a healthy cell. When expression of SCD1 in breast cancer patients was examined, results indicated that increased expression of SCD1 was associated with shorter patient survival <ref name="Holder">PMID: 23208590</ref>. This is most likely due to the promoter region of SCD1. A transcription factor named [https://en.wikipedia.org/wiki/Sterol_regulatory_element-binding_protein_1 SREBP-1] binds tightly to the promotor of SCD1, and both become upregulated when the PI3K signaling pathway is active<ref name="Holder" />. [https://cshperspectives.cshlp.org/content/4/9/a011189 PI3K/Akt signaling]is involved with cancer cell proliferation when the pathway becomes disrupted <ref name="Vara">PMID: 15023437</ref>. | SCD1 works to keep a balance between saturated and monosaturated fatty acids, and in turn, this helps prevent the build up of toxic saturated fats inside the cell<ref name="Holder" />. Increased expression levels of SCD1 has shown to enhance cancer cell proliferation. The concentration of monounsaturated fatty acids (MUFAs) is much higher in a cancer cell versus a healthy cell. When expression of SCD1 in breast cancer patients was examined, results indicated that increased expression of SCD1 was associated with shorter patient survival <ref name="Holder">PMID: 23208590</ref>. This is most likely due to the promoter region of SCD1. A transcription factor named [https://en.wikipedia.org/wiki/Sterol_regulatory_element-binding_protein_1 SREBP-1] binds tightly to the promotor of SCD1, and both become upregulated when the PI3K signaling pathway is active<ref name="Holder" />. [https://cshperspectives.cshlp.org/content/4/9/a011189 PI3K/Akt signaling]is involved with cancer cell proliferation when the pathway becomes disrupted <ref name="Vara">PMID: 15023437</ref>. | ||
Revision as of 02:24, 27 April 2021
Desaturation of Fatty Stearoyl-CoA by SCD1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers (Basel). 2019 Jul 5;11(7). pii: cancers11070948. doi:, 10.3390/cancers11070948. PMID:31284458 doi:http://dx.doi.org/10.3390/cancers11070948
- ↑ 3.0 3.1 3.2 3.3 Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
- ↑ Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, Li K, Sang BC. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat Struct Mol Biol. 2015 Jul;22(7):581-5. doi: 10.1038/nsmb.3049. Epub 2015 Jun , 22. PMID:26098317 doi:http://dx.doi.org/10.1038/nsmb.3049
- ↑ 5.0 5.1 Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006 Jun;116(6):1686-95. doi: 10.1172/JCI26991. PMID:16741579 doi:http://dx.doi.org/10.1172/JCI26991
- ↑ Yokoyama S, Hosoi T, Ozawa K. Stearoyl-CoA Desaturase 1 (SCD1) is a key factor mediating diabetes in MyD88-deficient mice. Gene. 2012 Apr 15;497(2):340-3. doi: 10.1016/j.gene.2012.01.024. Epub 2012 Feb 3. PMID:22326531 doi:http://dx.doi.org/10.1016/j.gene.2012.01.024
- ↑ Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11482-6. doi:, 10.1073/pnas.132384699. Epub 2002 Aug 12. PMID:12177411 doi:http://dx.doi.org/10.1073/pnas.132384699
- ↑ 8.0 8.1 8.2 Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do KA, Fraser Symmans W, Pusztai L, Hortobagyi GN, Mills GB, Meric-Bernstam F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat. 2013 Jan;137(1):319-27. doi: 10.1007/s10549-012-2354-4. , Epub 2012 Dec 4. PMID:23208590 doi:http://dx.doi.org/10.1007/s10549-012-2354-4
- ↑ Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004 Apr;30(2):193-204. doi: 10.1016/j.ctrv.2003.07.007. PMID:15023437 doi:http://dx.doi.org/10.1016/j.ctrv.2003.07.007
- ↑ Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017 Mar 2;20(3):303-314.e5. doi: 10.1016/j.stem.2016.11.004., Epub 2016 Dec 29. PMID:28041894 doi:http://dx.doi.org/10.1016/j.stem.2016.11.004
Student Contributors
- Brianna M. Avery
- William J. Harris III
- Emily M. Royston