We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Function == | == Function == | ||
| - | [[Image:Common_Eastern_Firefly.jpg|thumb|left|The Common Eastern Firefly in a hand emitting a yellow hue, showing bioluminescence.]] | + | [[Image:Common_Eastern_Firefly.jpg|thumb|left|upright=1.1|The Common Eastern Firefly in a hand emitting a yellow hue, showing bioluminescence.]] |
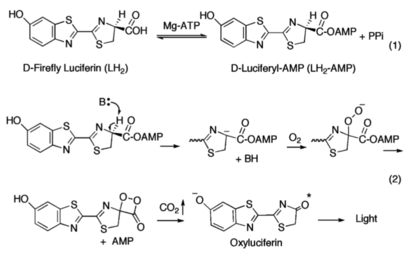
The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | ||
| Line 14: | Line 14: | ||
=== Biochemical Mechanism of LH2-AMP Oxidation=== | === Biochemical Mechanism of LH2-AMP Oxidation=== | ||
| - | [[Image:Mechanism_of_Firefly_Bioluminescence.png|thumb|The generally accepted mechanism of firefly bioluminescence. The first reaction involves the production of an luciferyl-adenylate intermediate (1). The second reaction involves oxidative decarboxylation that emits CO2 and results in bioluminescent properties.]] | + | [[Image:Mechanism_of_Firefly_Bioluminescence.png|thumb|upright=2.3|The generally accepted mechanism of firefly bioluminescence. The first reaction involves the production of an luciferyl-adenylate intermediate (1). The second reaction involves oxidative decarboxylation that emits CO2 and results in bioluminescent properties(2).]] |
Nothing but pain. | Nothing but pain. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 12:40, 27 April 2021
Firefly Luciferase
tttaaarrgggetttt to the right plaaccceee now the references are messed uppp and im dumb :(
| |||||||||||
References