We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
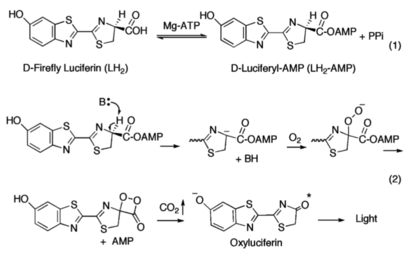
The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | ||
| - | ANL enzymes follow a domain alternation strategy for the first adenylation reaction, in which the reaction is catalyzed by one conformation and following the formation of the adenylate intermediate and release of pyrophosphate (PPi), the C-terminal domain undergoes a rotational transformation that is necessary for the second partial reaction. The active site of ANL enzymes resides between a 400-500 residue N-terminal domain and a smaller C-terminal domain of ~110-130 amino acids<ref name="Sundlov">Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. ''Biochemistry 51''(33), 6493-6495. https://doi.org/10.1021/bi300934s</ref>. Ten conserved regions of these proteins have been termed the A1-A10 motifs which play critical roles in either or both partial reactions. Two lysine residues are required for each partial reaction suggestive that luciferase similarly adopts a rotational transformation for complete catalysis. A mutation of Lys529, the A10 lysine, impairs only the initial adenylation reaction<ref name="Sundlov"/> whereas mutation of Lys443 in the A8 region disrupts the oxidative reaction<ref name="Sundlov"/>. | + | ANL enzymes follow a domain alternation strategy for the first adenylation reaction, in which the reaction is catalyzed by one conformation and following the formation of the adenylate intermediate and release of pyrophosphate (PPi), the C-terminal domain undergoes a rotational transformation that is necessary for the second partial reaction. The active site of ANL enzymes resides between a 400-500 residue N-terminal domain and a smaller C-terminal domain of ~110-130 amino acids<ref name="Sundlov">Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. ''Biochemistry 51''(33), 6493-6495. https://doi.org/10.1021/bi300934s</ref>. Ten conserved regions of these proteins have been termed the A1-A10 motifs which play critical roles in either or both partial reactions<ref name="Marahiel">Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. ''Chemical Reviews 97''(7), 2651-2674. https://doi.org/10.1021/cr960029e</ref>. Two lysine residues are required for each partial reaction suggestive that luciferase similarly adopts a rotational transformation for complete catalysis. A mutation of Lys529, the A10 lysine, impairs only the initial adenylation reaction<ref name="Sundlov"/> whereas mutation of Lys443 in the A8 region disrupts the oxidative reaction<ref name="Sundlov"/>. |
| Line 92: | Line 92: | ||
== References == | == References == | ||
<ref name="Sundlov"/> | <ref name="Sundlov"/> | ||
| - | + | <ref name="Marahiel"/> | |
<references/> | <references/> | ||
Revision as of 12:51, 27 April 2021
Firefly Luciferase
tttaaarrgggetttt to the right plaaccceee now the references are messed uppp and im dumb :(
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. Biochemistry 51(33), 6493-6495. https://doi.org/10.1021/bi300934s
- ↑ 2.0 2.1 Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. Chemical Reviews 97(7), 2651-2674. https://doi.org/10.1021/cr960029e