We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=== Conformation === | === Conformation === | ||
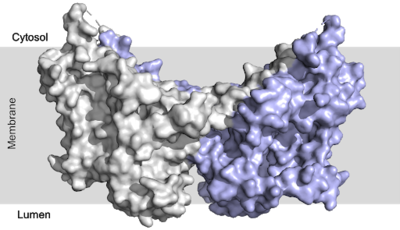
| - | + | Since DGAT1 is a transmembrane protein, it has 3 distinct regions: cytosolic, transmembrane, and luminal. Most of the enzyme exists within the membrane with small portions peeking out into the cytosol of the cell or [https://en.wikipedia.org/wiki/Lumen_(anatomy) lumen] of the endoplasmic reticulum. DGAT exists as a [https://en.wikipedia.org/wiki/Protein_dimer homodimer] of two identical chains, A and B. The homodimer interface is stabilized in two different ways. First, the transmembrane region is stabilized through large <scene name='87/877557/Hydrophobic_interface/3'>hydrophobic interactions</scene> between the TM1 helices on each of the chains. The second is through extensive <scene name='87/877557/Hydrophilic_interface/5'>hydrogen bonding interactions</scene> between the two chains in the cytosolic domain. <ref name="Sui">PMID:32433611</ref> <ref name="Wang">PMID:32433610</ref> | |
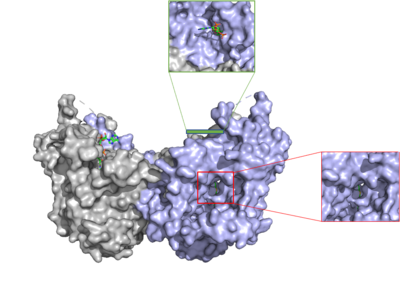
=== Tunnel System === | === Tunnel System === | ||
| Line 16: | Line 16: | ||
=== Active Site === | === Active Site === | ||
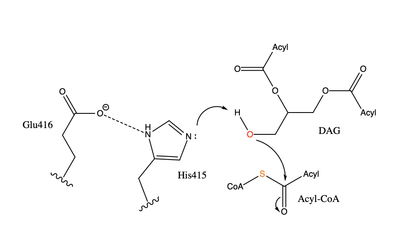
| - | The active site of | + | The active site of DGAT1 is in the transmembrane region of the enzyme. When it is in its <scene name='87/877557/His_no_oleoyl/6'>unbound</scene> state and no oleoyl-CoA is in its tunnel, Met434 hydrogen bonds to the catalytic Histidine, His415, which stabilizes the conformation. There are no major conformation changes that take place upon oleoyl-CoA binding into the cytosolic tunnel, however, several key residues change conformation to allow for the entrance of the ligand. In its <scene name='87/877557/Active_site/6'>bound</scene> conformation, His415 hydrogen bonds to Gln465 which stabilizes the Histidine and allows it to be positioned near the thioester bond of the oleoyl-CoA.<ref name="Sui">PMID:32433611</ref> The His415 interacts with the DAG that enters in a tunnel perpendicular to the oleoyl-CoA. <scene name='87/877557/Asn378/2'>Asn378</scene> has been hypothesized to be important in holding the DAG in a proper orientation to be able to interact with the oleoyl-CoA and become a triglyceride. <ref name="Sui">PMID:32433611</ref> |
=== Mechanism === | === Mechanism === | ||
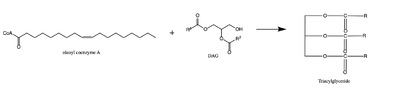
| - | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4: Arrow pushing mechanism of DGAT1 triglyceride synthesis]] When a molecule of | + | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4: Arrow pushing mechanism of DGAT1 triglyceride synthesis]] When a molecule of DAG, or another acyl acceptor, binds into this hydrophobic tunnel, DGAT1 transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride (Fig. 1). The catalytic histidine deprotonates the hydroxyl group on the C3 of the glycerol backbone (not shown). The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and the tetrahedral oleoyl-CoA-DAG intermediate is formed which is likely stabilized by Gln465. <ref name="Wang">Wang L;Qian H;Nian Y;Han Y;Ren Z;Zhang H;Hu L;Prasad BVV;Laganowsky A;Yan N;Zhou M;. (2020, May 13). Structure and mechanism of human diacylglycerol o-acyltransferase 1. Retrieved March 09, 2021, from https://pubmed.ncbi.nlm.nih.gov/32433610/</ref> The electron density then falls back down to the [https://en.wikipedia.org/wiki/Carbonyl_group carbonyl] carbon and to the sulfur of the oleoyl-CoA which accepts the added electron density and the bond between the sulfur and carbonyl carbon is broken. (Fig. 4) A proposed interaction between <scene name='87/878228/415_416_465_with_oleoyl_coa/4'>Glu416</scene> and His415 likely provides enhancement of the reaction by deprotonating His415 which shifts the electron density and helps facilitate the deprotonation of the glycerol backbone. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 and Glu416 support the hypothesis that it is essential for catalysis in the active site since the enzyme function was completely eliminated when the mutation was made. <ref name="Wang">PMID:32433610</ref> |
==Regulation== | ==Regulation== | ||
Revision as of 13:56, 27 April 2021
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
[1] [10] [11] [12] [9] [13] [2] [4] [3]
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Caldo, K., Acedo, J. Z., Panigrahi, R., Vederas, J. C., Weselake, R. J., & Lemieux, M. J. (2017). Diacylglycerol Acyltransferase 1 Is Regulated by Its N-Terminal Domain in Response to Allosteric Effectors. Plant physiology, 175(2), 667–680. https://doi.org/10.1104/pp.17.00934
- ↑ Denison, H., Nilsson, C., Löfgren, L., Himmelmann, A., Mårtensson, G., Knutsson, M., Al-Shurbaji, A., Tornqvist, H., & Eriksson, J. W. (2014). Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes, obesity & metabolism, 16(4), 334–343. https://doi.org/10.1111/dom.12221
- ↑ Cao, J., Zhou, Y., Peng, H., Huang, X., Stahler, S., Suri, V., Qadri, A., Gareski, T., Jones, J., Hahm, S., Perreault, M., McKew, J., Shi, M., Xu, X., Tobin, J. F., & Gimeno, R. E. (2011). Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. The Journal of biological chemistry, 286(48), 41838–41851. https://doi.org/10.1074/jbc.M111.245456
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
- ↑ 9.0 9.1 Gluchowski, N. L., Chitraju, C., Picoraro, J. A., Mejhert, N., Pinto, S., Xin, W., Kamin, D. S., Winter, H. S., Chung, W. K., Walther, T. C., & Farese, R. V., Jr (2017). Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. Journal of lipid research, 58(6), 1230–1237. https://doi.org/10.1194/jlr.P075119
- ↑ Caldo, K., Acedo, J. Z., Panigrahi, R., Vederas, J. C., Weselake, R. J., & Lemieux, M. J. (2017). Diacylglycerol Acyltransferase 1 Is Regulated by Its N-Terminal Domain in Response to Allosteric Effectors. Plant physiology, 175(2), 667–680. https://doi.org/10.1104/pp.17.00934
- ↑ Cao, J., Zhou, Y., Peng, H., Huang, X., Stahler, S., Suri, V., Qadri, A., Gareski, T., Jones, J., Hahm, S., Perreault, M., McKew, J., Shi, M., Xu, X., Tobin, J. F., & Gimeno, R. E. (2011). Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. The Journal of biological chemistry, 286(48), 41838–41851. https://doi.org/10.1074/jbc.M111.245456
- ↑ Denison, H., Nilsson, C., Löfgren, L., Himmelmann, A., Mårtensson, G., Knutsson, M., Al-Shurbaji, A., Tornqvist, H., & Eriksson, J. W. (2014). Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes, obesity & metabolism, 16(4), 334–343. https://doi.org/10.1111/dom.12221
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko