We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
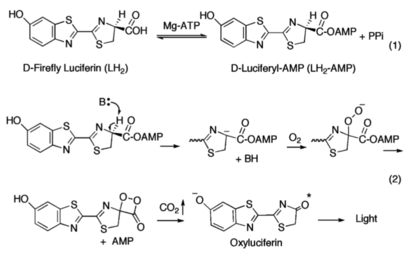
(going through changes but math is cool :/)The A8 motif harbors the hinge residue at Lys439 and the antiparallel two stranded β-sheet is directed into the active site of the enzyme. The φ/ψ angles of Lys439 change from −73°/−12° in the structure of wild-type luciferase in the adenylate-forming conformation to −69°/158° in the cross- linked structure.this illustrates that a large component of the conformational change occurs with a rotation of the ψ angle of the hinge residue. Additional torsion angle changes are seen in φ angles for Arg437 and Leu441, although the magnitude of the change is not as large as at the hinge residue Lys439<ref name="Sundlov"/>. | (going through changes but math is cool :/)The A8 motif harbors the hinge residue at Lys439 and the antiparallel two stranded β-sheet is directed into the active site of the enzyme. The φ/ψ angles of Lys439 change from −73°/−12° in the structure of wild-type luciferase in the adenylate-forming conformation to −69°/158° in the cross- linked structure.this illustrates that a large component of the conformational change occurs with a rotation of the ψ angle of the hinge residue. Additional torsion angle changes are seen in φ angles for Arg437 and Leu441, although the magnitude of the change is not as large as at the hinge residue Lys439<ref name="Sundlov"/>. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 14:20, 27 April 2021
Firefly Luciferase
tttaaarrgggetttt to the right plaaccceee and finishh :(
| |||||||||||
References
- ↑ Branchini, B. R., Magyar, R. A., Murtiashaw, M. H., Anderson, S. M., Helgerson, L. C., & Zimmer, M. (1999). Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color. Biochemistry 38(40), 13223–13230. https://doi.org/10.1021/bi991181o
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. Biochemistry 51(33), 6493-6495. https://doi.org/10.1021/bi300934s
- ↑ Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. Chemical Reviews 97(7), 2651-2674. https://doi.org/10.1021/cr960029e
- ↑ Branchini, B. R., Murtiashaw, M. H., Magyar, R. A., Anderson, S. M. (2000). The Role of Lysine 529, a Conserved Residue of the Acyl-Adenylate-Forming Enzyme Superfamily, in Firefly Luciferase. Biochemistry 39(18), 5433-5440. https://doi.org/10.1021/bi9928804