We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
==Regulation== | ==Regulation== | ||
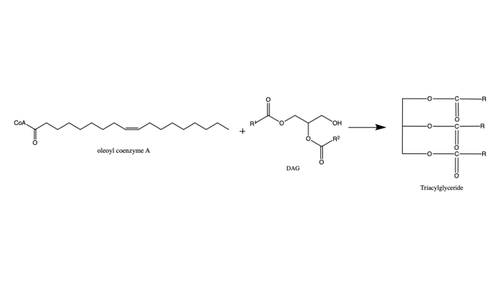
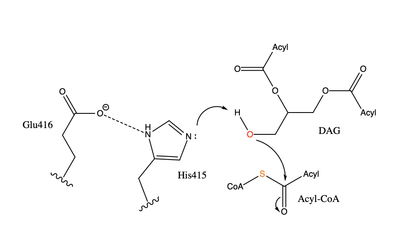
| - | Regulation of DGAT1 is thought to be performed by the hydrophilic N-terminal domain which regulates activity based on acyl-CoA/CoA levels. The N-terminal domain contains an intrinsically disordered region and a folded segment. The disordered region has an autoinhibitory function and a dimerization interface, and the folded segment was found to have an allosteric site of acyl-CoA/CoA. When acyl-CoA levels increase, the binding of acyl-CoA with this non-catalytic site initiates allosteric activation. Enzyme activation is prevented until limiting acyl-CoA conditions, which allows CoA to act as a noncompetitive feedback inhibitor. For these reasons, it is proposed that the N-terminal domain of DGAT1 acts as a positive and negative regulator. <ref name= | + | Regulation of DGAT1 is thought to be performed by the hydrophilic N-terminal domain which regulates activity based on acyl-CoA/CoA levels. The N-terminal domain contains an intrinsically disordered region and a folded segment. The disordered region has an autoinhibitory function and a dimerization interface, and the folded segment was found to have an allosteric site of acyl-CoA/CoA. When acyl-CoA levels increase, the binding of acyl-CoA with this non-catalytic site initiates allosteric activation. Enzyme activation is prevented until limiting acyl-CoA conditions, which allows CoA to act as a noncompetitive feedback inhibitor. For these reasons, it is proposed that the N-terminal domain of DGAT1 acts as a positive and negative regulator. <ref name="Caldo">doi.org/10.1104/pp.17.00934</ref> |
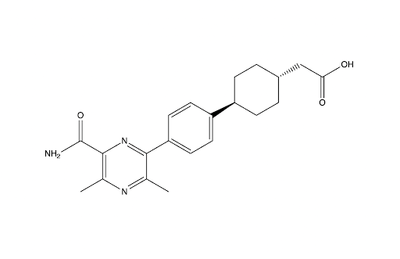
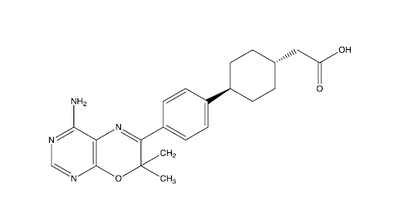
==Inhibitors== | ==Inhibitors== | ||
Revision as of 14:35, 27 April 2021
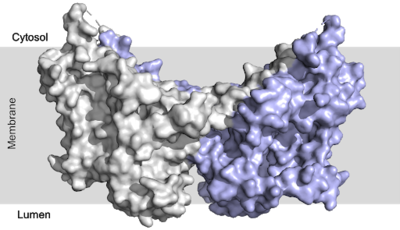
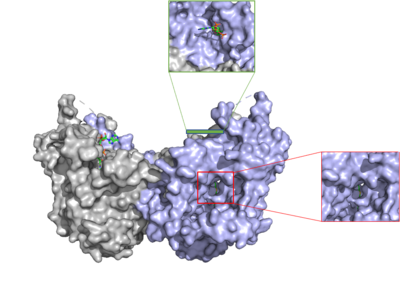
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
[1] [10] [11] [12] [9] [13] [2] [4] [3]
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ doi.org/10.1104/pp.17.00934
- ↑ Denison, H., Nilsson, C., Löfgren, L., Himmelmann, A., Mårtensson, G., Knutsson, M., Al-Shurbaji, A., Tornqvist, H., & Eriksson, J. W. (2014). Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes, obesity & metabolism, 16(4), 334–343. https://doi.org/10.1111/dom.12221
- ↑ Cao, J., Zhou, Y., Peng, H., Huang, X., Stahler, S., Suri, V., Qadri, A., Gareski, T., Jones, J., Hahm, S., Perreault, M., McKew, J., Shi, M., Xu, X., Tobin, J. F., & Gimeno, R. E. (2011). Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. The Journal of biological chemistry, 286(48), 41838–41851. https://doi.org/10.1074/jbc.M111.245456
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
- ↑ 9.0 9.1 Gluchowski, N. L., Chitraju, C., Picoraro, J. A., Mejhert, N., Pinto, S., Xin, W., Kamin, D. S., Winter, H. S., Chung, W. K., Walther, T. C., & Farese, R. V., Jr (2017). Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. Journal of lipid research, 58(6), 1230–1237. https://doi.org/10.1194/jlr.P075119
- ↑ Caldo, K., Acedo, J. Z., Panigrahi, R., Vederas, J. C., Weselake, R. J., & Lemieux, M. J. (2017). Diacylglycerol Acyltransferase 1 Is Regulated by Its N-Terminal Domain in Response to Allosteric Effectors. Plant physiology, 175(2), 667–680. https://doi.org/10.1104/pp.17.00934
- ↑ Cao, J., Zhou, Y., Peng, H., Huang, X., Stahler, S., Suri, V., Qadri, A., Gareski, T., Jones, J., Hahm, S., Perreault, M., McKew, J., Shi, M., Xu, X., Tobin, J. F., & Gimeno, R. E. (2011). Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. The Journal of biological chemistry, 286(48), 41838–41851. https://doi.org/10.1074/jbc.M111.245456
- ↑ Denison, H., Nilsson, C., Löfgren, L., Himmelmann, A., Mårtensson, G., Knutsson, M., Al-Shurbaji, A., Tornqvist, H., & Eriksson, J. W. (2014). Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes, obesity & metabolism, 16(4), 334–343. https://doi.org/10.1111/dom.12221
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko