We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Kaitlyn Roberts/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
=== Active Site === | === Active Site === | ||
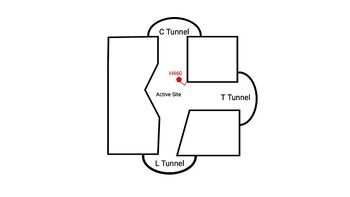
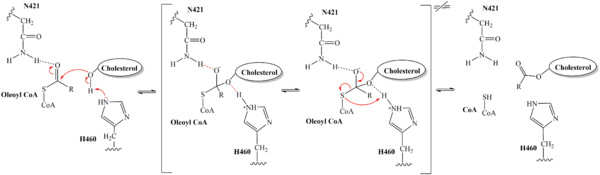
| - | Within the binding pocket, there are several <scene name='87/ | + | Within the binding pocket, there are several <scene name='87/877561/Important_residues_2_final/1'>highly conserved residues</scene>. Their high preservation suggests that the local environment of the binding pocket plays a major role in SOAT activity, but their specific interactions are currently not well studied. However, <scene name='87/877561/Important_residues_final/1'>W420, N421, and H460</scene> have been identified as key catalytic residues.<ref name="Guan" /> Histidine, commonly used as the catalytic base to initiate acyl transferase reactions, is assumed to be the most important catalytic residue in SOAT.<ref name="Das">PMID:18480028</ref> This was confirmed as mutating H460 to alanine completely abolished enzymatic activity.<ref name="Guo">PMID:16154994</ref> Additionally, H460 is highly conserved across a variety of species, further emphasizing its importance in SOAT catalysis.<ref name="Guan" /> It is hypothesized that N421 stabalizes the transition state via hydrogen bonding with coenzyme A.<ref name="Qian" /> Additionally, mutations of W420 to alanine render SOAT nonfunctional, indicating that it must be essential for catalytic activity. However, its role in the mechanism is not explicitly hypothesized. We believe that it plays a role in substrate binding through <scene name='87/879459/W420_intx/3'>hydrophobic interactions</scene> with the acyl chain of coenzyme A. |
=== Catalytic Mechanism === | === Catalytic Mechanism === | ||
Revision as of 15:33, 27 April 2021
Human Sterol O-acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 2.0 2.1 2.2 2.3 Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Das A, Davis MA, Rudel LL. Identification of putative active site residues of ACAT enzymes. J Lipid Res. 2008 Aug;49(8):1770-81. doi: 10.1194/jlr.M800131-JLR200. Epub 2008, May 13. PMID:18480028 doi:http://dx.doi.org/10.1194/jlr.M800131-JLR200
- ↑ Guo ZY, Lin S, Heinen JA, Chang CC, Chang TY. The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J Biol Chem. 2005 Nov 11;280(45):37814-26. doi: 10.1074/jbc.M508384200. Epub 2005, Sep 8. PMID:16154994 doi:http://dx.doi.org/10.1074/jbc.M508384200
- ↑ 5.0 5.1 Bhattacharyya R, Kovacs DM. ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta. 2010 Aug;1801(8):960-5. doi: 10.1016/j.bbalip.2010.04.003. , Epub 2010 Apr 14. PMID:20398792 doi:http://dx.doi.org/10.1016/j.bbalip.2010.04.003
- ↑ 6.0 6.1 Huttunen HJ, Kovacs DM. ACAT as a drug target for Alzheimer's disease. Neurodegener Dis. 2008;5(3-4):212-4. doi: 10.1159/000113705. Epub 2008 Mar 6. PMID:18322393 doi:http://dx.doi.org/10.1159/000113705
- ↑ Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2006 Mar;38(3):151-6. doi:, 10.1111/j.1745-7270.2006.00154.x. PMID:16518538 doi:http://dx.doi.org/10.1111/j.1745-7270.2006.00154.x
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
Student Contributors
- Kylie Pfeifer
- Stephanie Pellegrino
- Kaitlyn Roberts