We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Hannah Wright/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
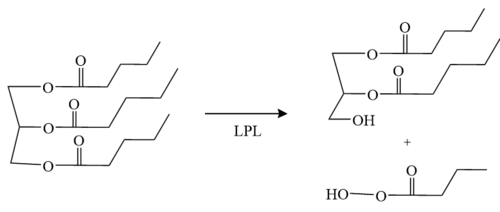
The C-terminus of LPL consists of 12 β-sheets and is known as the <scene name='87/877603/B-domain/2'>C-terminal flattened β-barrel domain</scene>. The β-sheets are interacting giving a shape that resembles an elongated cylinder or barrel<ref name="Wong">PMID:8144612</ref>. | The C-terminus of LPL consists of 12 β-sheets and is known as the <scene name='87/877603/B-domain/2'>C-terminal flattened β-barrel domain</scene>. The β-sheets are interacting giving a shape that resembles an elongated cylinder or barrel<ref name="Wong">PMID:8144612</ref>. | ||
====Interaction of LPL and GPIHBP1==== | ====Interaction of LPL and GPIHBP1==== | ||
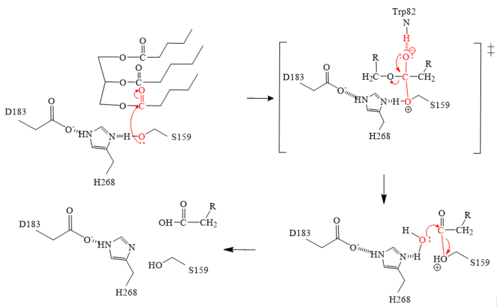
| - | GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the hydrophobic effect and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL<ref name="Birrane">PMID:30559189</ref>. | + | GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic effect] and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL<ref name="Birrane">PMID:30559189</ref>. |
====Calcium Ion Coordination==== | ====Calcium Ion Coordination==== | ||
The <scene name='87/877636/Calcium_ion_coordination/1'>calcium ion</scene> has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the coordinating residues can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. | The <scene name='87/877636/Calcium_ion_coordination/1'>calcium ion</scene> has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the coordinating residues can give rise to detrimental metabolic diseases. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. | ||
Revision as of 16:16, 27 April 2021
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ 2.0 2.1 Wong H, Davis RC, Thuren T, Goers JW, Nikazy J, Waite M, Schotz MC. Lipoprotein lipase domain function. J Biol Chem. 1994 Apr 8;269(14):10319-23. PMID:8144612
- ↑ Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
- ↑ 4.0 4.1 Falko JM. Familial Chylomicronemia Syndrome: A Clinical Guide For Endocrinologists. Endocr Pract. 2018 Aug;24(8):756-763. doi: 10.4158/EP-2018-0157. PMID:30183397 doi:http://dx.doi.org/10.4158/EP-2018-0157
Student/Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright