User:Hannah Wright/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
===LPL=== | ===LPL=== | ||
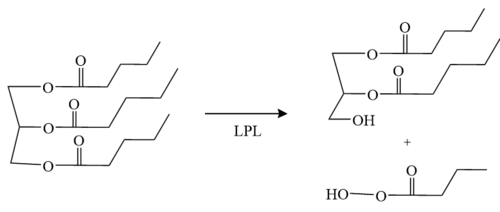
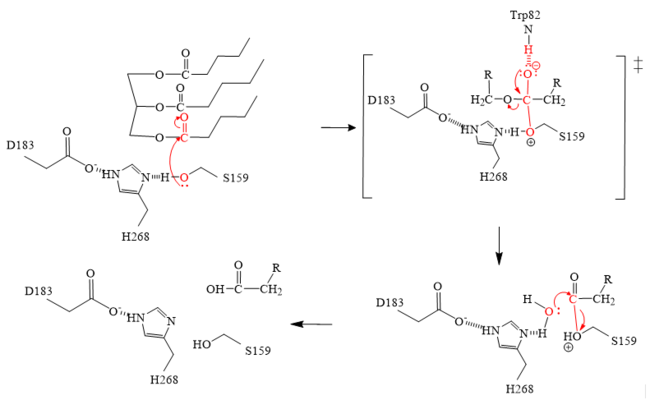
LPL has two main domains, the larger N-terminus domain containing the active site and the smaller C-terminus domain. These two domains are connected via a peptide linker hinge. LPL also contains a large basic patch and a single calcium ion. Additionally, LPL consists of two N-linked [http://en.wikipedia.org/wiki/Glycan glycans] (N70, N386) which likely contribute to the correct folding of LPL due to the attached [http://en.wikipedia.org/wiki/Oligosaccharide oligosaccharides]. Five [http://en.wikipedia.org/wiki/Disulfide disulfide bonds] contribute to the stabilization throughout LPL’s structure. Lastly, the active site in the larger N-terminus domain is lined with hydrophobic residues <ref name="Birrane">PMID:30559189</ref>. | LPL has two main domains, the larger N-terminus domain containing the active site and the smaller C-terminus domain. These two domains are connected via a peptide linker hinge. LPL also contains a large basic patch and a single calcium ion. Additionally, LPL consists of two N-linked [http://en.wikipedia.org/wiki/Glycan glycans] (N70, N386) which likely contribute to the correct folding of LPL due to the attached [http://en.wikipedia.org/wiki/Oligosaccharide oligosaccharides]. Five [http://en.wikipedia.org/wiki/Disulfide disulfide bonds] contribute to the stabilization throughout LPL’s structure. Lastly, the active site in the larger N-terminus domain is lined with hydrophobic residues <ref name="Birrane">PMID:30559189</ref>. | ||
| - | ===GPIHBP1=== | ||
| - | GPIHBP1 is a <scene name='87/877603/3-fingered/2'>three-fingered</scene> LU (Ly6/uPAR) domain. GPIHBP1 is stabilized by five disulfide bonds and is also known for its N-terminal intrinsically disordered acidic domain<ref name="Birrane">PMID:30559189</ref>. The N-terminal intrinsically disordered acidic domain (residues 21-61) has not been resolved experimentally and is not shown in the crystal structure. | ||
| - | ===LPL GPIHBP1 complex=== | ||
====N-terminus of LPL==== | ====N-terminus of LPL==== | ||
The N-terminus of LPL is made up of 6 [http://en.wikipedia.org/wiki/Alpha_helix α-helices] and 10 [http://en.wikipedia.org/wiki/Beta_sheet β-sheets] known as the <scene name='87/877603/A-b_domain/1'>N-terminal α/β-hydrolase domain</scene>. Additionally, α/β hydrolase harbors the catalytic triad<ref name="Wong">PMID:8144612</ref>. | The N-terminus of LPL is made up of 6 [http://en.wikipedia.org/wiki/Alpha_helix α-helices] and 10 [http://en.wikipedia.org/wiki/Beta_sheet β-sheets] known as the <scene name='87/877603/A-b_domain/1'>N-terminal α/β-hydrolase domain</scene>. Additionally, α/β hydrolase harbors the catalytic triad<ref name="Wong">PMID:8144612</ref>. | ||
====C-terminus of LPL==== | ====C-terminus of LPL==== | ||
The C-terminus of LPL consists of 12 β-sheets and is known as the <scene name='87/877603/B-domain/2'>C-terminal flattened β-barrel domain</scene>. The β-sheets are interacting giving a shape that resembles an elongated cylinder or barrel<ref name="Wong">PMID:8144612</ref>. | The C-terminus of LPL consists of 12 β-sheets and is known as the <scene name='87/877603/B-domain/2'>C-terminal flattened β-barrel domain</scene>. The β-sheets are interacting giving a shape that resembles an elongated cylinder or barrel<ref name="Wong">PMID:8144612</ref>. | ||
| + | ===GPIHBP1=== | ||
| + | GPIHBP1 is a <scene name='87/877603/3-fingered/2'>three-fingered</scene> LU (Ly6/uPAR) domain. GPIHBP1 is stabilized by five disulfide bonds and is also known for its N-terminal intrinsically disordered acidic domain<ref name="Birrane">PMID:30559189</ref>. The N-terminal intrinsically disordered acidic domain (residues 21-61) has not been resolved experimentally and is not shown in the crystal structure. | ||
| + | ===LPL-GPIHBP1 complex=== | ||
====Interaction of LPL and GPIHBP1==== | ====Interaction of LPL and GPIHBP1==== | ||
GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic effect] and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL<ref name="Birrane">PMID:30559189</ref>. | GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877603/Lpl_gpihb1_interaction/3'>hydrophobic interactions</scene>. This is largely due to the [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic effect] and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL<ref name="Birrane">PMID:30559189</ref>. | ||
Revision as of 19:32, 27 April 2021
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ 2.0 2.1 Wong H, Davis RC, Thuren T, Goers JW, Nikazy J, Waite M, Schotz MC. Lipoprotein lipase domain function. J Biol Chem. 1994 Apr 8;269(14):10319-23. PMID:8144612
- ↑ Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
- ↑ 4.0 4.1 Falko JM. Familial Chylomicronemia Syndrome: A Clinical Guide For Endocrinologists. Endocr Pract. 2018 Aug;24(8):756-763. doi: 10.4158/EP-2018-0157. PMID:30183397 doi:http://dx.doi.org/10.4158/EP-2018-0157
Student/Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright