We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Fleshman/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
Cholesterol esters were found in arterial lesions in 1910, but the first ACAT1 activity was discovered in the mid 1900's. This led to the inhibition of ACAT1 as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT1 inhibitors grew, but some of the compounds developed exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT1 gene was successfully cloned. This discovery led to more studies with ACAT1 and atherosclerosis. Some of these studies used mice and also showed cellular toxicity. ACAT1 inhibition is still being pursued as a strategy for treatment or prevention of atherosclerosis and related diseases. | Cholesterol esters were found in arterial lesions in 1910, but the first ACAT1 activity was discovered in the mid 1900's. This led to the inhibition of ACAT1 as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT1 inhibitors grew, but some of the compounds developed exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT1 gene was successfully cloned. This discovery led to more studies with ACAT1 and atherosclerosis. Some of these studies used mice and also showed cellular toxicity. ACAT1 inhibition is still being pursued as a strategy for treatment or prevention of atherosclerosis and related diseases. | ||
<ref name="Farese Jr.">PMID: 16857957</ref> | <ref name="Farese Jr.">PMID: 16857957</ref> | ||
| - | |||
==Structural Overview== | ==Structural Overview== | ||
| Line 22: | Line 21: | ||
===Dimer-Dimer Interface=== | ===Dimer-Dimer Interface=== | ||
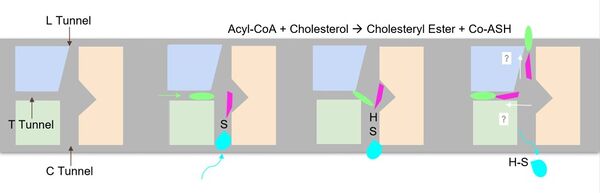
The <scene name='87/877604/Dimer_interface/19'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner <ref name="Guan">PMID:32424158</ref>. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of Acyl-CoA and cholesterol induce conformational changes that activate the tunnels necessary for substrates to enter them. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway <ref name="Qian">PMID:32433614</ref>. | The <scene name='87/877604/Dimer_interface/19'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner <ref name="Guan">PMID:32424158</ref>. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of Acyl-CoA and cholesterol induce conformational changes that activate the tunnels necessary for substrates to enter them. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway <ref name="Qian">PMID:32433614</ref>. | ||
| + | |||
| + | |||
| Line 38: | Line 39: | ||
The <scene name='87/877605/T_tunnel/3'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important <scene name='87/877605/T_tunnel_residues/3'>residues of the T tunnel</scene> include Arg262, Phe263, and Leu306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism. Upon mutation of these residues, the tunnel function was inhibited. <ref name="Qian">PMID:32433614</ref> | The <scene name='87/877605/T_tunnel/3'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important <scene name='87/877605/T_tunnel_residues/3'>residues of the T tunnel</scene> include Arg262, Phe263, and Leu306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism. Upon mutation of these residues, the tunnel function was inhibited. <ref name="Qian">PMID:32433614</ref> | ||
The <scene name='87/877605/L_tunnel/4'>L tunnel</scene> provides a potential opening to the lumen side. The enzymatic reaction occurs at the intersection of these two tunnels. It is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol by way of the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen. The specific mechanism by which the cholesterol ester product exits the tunnel to enter the lumen has not yet been determined. | The <scene name='87/877605/L_tunnel/4'>L tunnel</scene> provides a potential opening to the lumen side. The enzymatic reaction occurs at the intersection of these two tunnels. It is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol by way of the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen. The specific mechanism by which the cholesterol ester product exits the tunnel to enter the lumen has not yet been determined. | ||
| - | |||
| - | |||
===Active Site=== | ===Active Site=== | ||
| Line 48: | Line 47: | ||
==Mechanism== | ==Mechanism== | ||
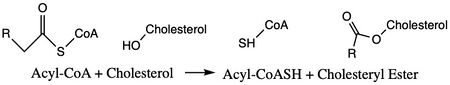
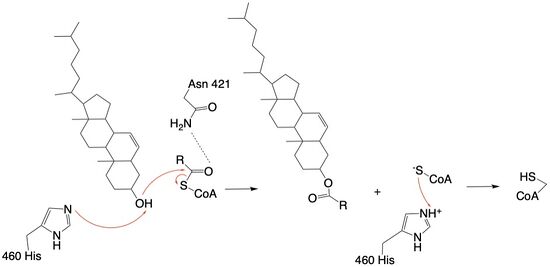
The mechanism of the acyltransferase reaction occurs in the catalytic site of each monomer in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (Figure 4). The His460 is positioned to deprotonate the cholesterol upon entering through the T tunnel: Acyl-CoA upon entering is positioned to where the sulfur bonded to the carboxyl carbon is at the direct intersection of the T tunnel into the active site. The Acyl CoA is held in place by the <scene name='87/877605/Catalytic_residues/9'>oxyanion hole</scene> of Asn421. This mechanism produces Acyl-CoASH and cholesteryl ester. The Acyl-CoASH leaves through the C tunnel to the cytosol. <ref name="Qian">PMID:32433614</ref> | The mechanism of the acyltransferase reaction occurs in the catalytic site of each monomer in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (Figure 4). The His460 is positioned to deprotonate the cholesterol upon entering through the T tunnel: Acyl-CoA upon entering is positioned to where the sulfur bonded to the carboxyl carbon is at the direct intersection of the T tunnel into the active site. The Acyl CoA is held in place by the <scene name='87/877605/Catalytic_residues/9'>oxyanion hole</scene> of Asn421. This mechanism produces Acyl-CoASH and cholesteryl ester. The Acyl-CoASH leaves through the C tunnel to the cytosol. <ref name="Qian">PMID:32433614</ref> | ||
| + | |||
| + | |||
Revision as of 20:29, 27 April 2021
Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1): Function, Structure, and Inhibition
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ 3.0 3.1 3.2 3.3 3.4 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
- ↑ Vaziri ND, Liang KH. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004 Jul 27;110(4):419-25. doi: 10.1161/01.CIR.0000136023.70841.0F. , Epub 2004 Jul 19. PMID:15262831 doi:http://dx.doi.org/10.1161/01.CIR.0000136023.70841.0F
- ↑ Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1262-7. doi: 10.1073/pnas.0336398100., Epub 2003 Jan 21. PMID:12538880 doi:http://dx.doi.org/10.1073/pnas.0336398100
- ↑ 7.0 7.1 7.2 7.3 Chang TY, Chang CC, Bryleva E, Rogers MA, Murphy SR. Neuronal cholesterol esterification by ACAT1 in Alzheimer's disease. IUBMB Life. 2010 Apr;62(4):261-7. doi: 10.1002/iub.305. PMID:20101629 doi:http://dx.doi.org/10.1002/iub.305
- ↑ 8.0 8.1 Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. PMID:26669800 doi:http://dx.doi.org/10.4155/fmc.15.161
Student Contributors
- Megan Fleshman, Tori Templin, Haylie Moehlenkamp