We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Betsy Johns/Sandbox 1
From Proteopedia
< User:Betsy Johns(Difference between revisions)
| Line 25: | Line 25: | ||
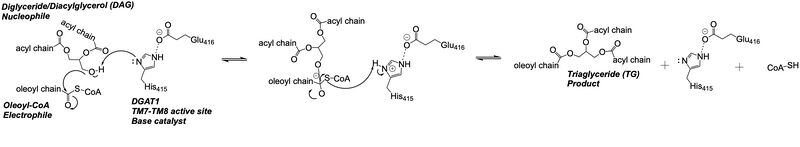
Depiction of the DGAT1 active site mechanism is shown in Figure 2. Oleoyl-CoA, the 18 carbon chain derivative of Acyl-CoA, was used to model the substrate Acyl-CoA.<ref name="Wang">PMID: 32433610</ref><ref name="Sui">PMID: 32433611</ref> DAG enters the active site of DGAT1 through its lateral gate while the catalytic His415 flips from the cytosolic side to the lumenal side, in order for the channel opening located on the cytosolic side of DGAT1 to widen enough to accommodate the Oleoyl-CoA.<ref name="Sui">PMID: 32433611</ref> The Oleoyl CoA then enters the active site through the channel opening located on the cytosolic side of DGAT1. When the Oleoyl-CoA and DAG are within close proximity in the active site, the catalytic His415 catalyzes the reaction.<ref name="Wang">PMID: 32433610</ref><ref name="Sui">PMID: 32433611</ref> The coenzyme A moiety exits through the channel located on the cytosolic side, while the product triacylglyceride exits through the lateral gate. | Depiction of the DGAT1 active site mechanism is shown in Figure 2. Oleoyl-CoA, the 18 carbon chain derivative of Acyl-CoA, was used to model the substrate Acyl-CoA.<ref name="Wang">PMID: 32433610</ref><ref name="Sui">PMID: 32433611</ref> DAG enters the active site of DGAT1 through its lateral gate while the catalytic His415 flips from the cytosolic side to the lumenal side, in order for the channel opening located on the cytosolic side of DGAT1 to widen enough to accommodate the Oleoyl-CoA.<ref name="Sui">PMID: 32433611</ref> The Oleoyl CoA then enters the active site through the channel opening located on the cytosolic side of DGAT1. When the Oleoyl-CoA and DAG are within close proximity in the active site, the catalytic His415 catalyzes the reaction.<ref name="Wang">PMID: 32433610</ref><ref name="Sui">PMID: 32433611</ref> The coenzyme A moiety exits through the channel located on the cytosolic side, while the product triacylglyceride exits through the lateral gate. | ||
| - | [[Image:Screen_Shot_2021-04-27_at_4.59.44_PM.png|800 px|center|thumb|'''Figure 2: Active Site Mechanism Overview''' Modeled is the active site of DGAT1 (shown in teal) with its catalytic Histidine (His415), Oleoyl CoA (shown in pink), and a general diacylglycerol (DAG, shown in yellow).]] | + | [[Image:Screen_Shot_2021-04-27_at_4.59.44_PM.png|800 px|center|thumb|'''Figure 2: Active Site Mechanism Overview''' Modeled is the active site of one subunit of DGAT1 (shown in teal) with its catalytic Histidine (His415), Oleoyl CoA (shown in pink), and a general diacylglycerol (DAG, shown in yellow). Shown are the substrates Oleoyl-CoA and DAG entering the active site of DGAT1, being catalyzed by His415, and then leaving through the channel they entered.]] |
=== Oleoyl-CoA Binding === | === Oleoyl-CoA Binding === | ||
Current revision
Diacylglycerol acyltransferase 1, DGAT1, synthesizes triacylglycerides
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 3.2 Ma D, Wang Z, Merrikh CN, Lang KS, Lu P, Li X, Merrikh H, Rao Z, Xu W. Crystal structure of a membrane-bound O-acyltransferase. Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct, 3. PMID:30283133 doi:http://dx.doi.org/10.1038/s41586-018-0568-2
- ↑ 4.0 4.1 4.2 4.3 Denison H, Nilsson C, Lofgren L, Himmelmann A, Martensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014 Apr;16(4):334-43. doi: 10.1111/dom.12221. Epub 2013 Oct, 31. PMID:24118885 doi:http://dx.doi.org/10.1111/dom.12221
- ↑ 5.0 5.1 Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, Farese RV Jr. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009 Aug;50(2):434-42. doi: 10.1002/hep.22980. PMID:19472314 doi:http://dx.doi.org/10.1002/hep.22980
- ↑ Stephen J, Vilboux T, Haberman Y, Pri-Chen H, Pode-Shakked B, Mazaheri S, Marek-Yagel D, Barel O, Di Segni A, Eyal E, Hout-Siloni G, Lahad A, Shalem T, Rechavi G, Malicdan MC, Weiss B, Gahl WA, Anikster Y. Congenital protein losing enteropathy: an inborn error of lipid metabolism due to DGAT1 mutations. Eur J Hum Genet. 2016 Aug;24(9):1268-73. doi: 10.1038/ejhg.2016.5. Epub 2016 Feb , 17. PMID:26883093 doi:http://dx.doi.org/10.1038/ejhg.2016.5
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa