User:Giselle Flores/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ||
=Lipoprotein Lipase coupled with GPIHBP1= | =Lipoprotein Lipase coupled with GPIHBP1= | ||
<StructureSection load='6ob0' size='350' side='right' caption='Lipoprotein Lipase PDB' scene='87/877513/Original_scene/1'> | <StructureSection load='6ob0' size='350' side='right' caption='Lipoprotein Lipase PDB' scene='87/877513/Original_scene/1'> | ||
| - | It should be noted that the PDB: 6OB0 was used to synthesize the following images. | ||
==Introduction== | ==Introduction== | ||
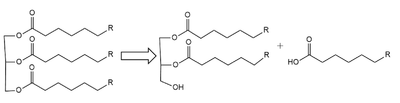
| - | <scene name='87/877513/Original_scene/1'>Lipoprotein Lipase</scene>(LPL) is an important enzyme for the breakdown of triglycerides in the body (Figure 1).<ref name=”Arora”>PMID:31072929</ref> [[Image:Simple_mech.png|400 px|right|thumb|Figure 1: breakdown of a triglyceride into a diglyceride and creation of one free fatty acid by LPL]] A [ | + | |
| + | It should be noted that the PDB: 6OB0 was used to synthesize the following images. | ||
| + | |||
| + | <scene name='87/877513/Original_scene/1'>Lipoprotein Lipase</scene>(LPL) is an important enzyme for the breakdown of triglycerides in the body (Figure 1).<ref name=”Arora”>PMID:31072929</ref> [[Image:Simple_mech.png|400 px|right|thumb|Figure 1: breakdown of a triglyceride into a diglyceride and creation of one free fatty acid by LPL]] A [http://en.wikipedia.org/wiki/Lipase lipase] is an enzyme that is capable of catalyzing the [http://en.wikipedia.org/wiki/Hydrolysis hydrolysis] of fats/lipids which are consumed through oils. It is encoded by the [http://www.genecards.org/cgi-bin/carddisp.pl?gene=LPL p22 region in chromosome 8]. Once synthesized, it is secreted into the interstitial space in several tissues. The main site of action for <scene name='87/877513/Original_scene/1'>LPL</scene> is in the [http://www.pnas.org/content/pnas/116/5/1480/F1.large.jpg capillary lumen] within muscle and adipose tissues.<ref name=”Birrane”>PMID:30559189</ref> The function of this lipase is to hydrolyze [http://en.wikipedia.org/wiki/Triglyceride triglycerides] of very-low-density lipoproteins ([http://qph.fs.quoracdn.net/main-qimg-8e874e647baeb69b00203c47165247e2 VLDL]) and to aid in the delivery of lipid nutrients to vital tissues.<ref name=”Birrane”>PMID:30559189</ref> The enzyme is commonly found on the surface of cells that line blood capillaries. Two different lipoproteins are essential to break down triglycerides. One of the lipoproteins is utilized to transport fat into the bloodstream from different organs.<ref name=”Arora”>PMID:31072929</ref> The lipoproteins essential, in the transport of fat from the intestine are referred to as [http://en.wikipedia.org/wiki/Chylomicron chylomicrons]. VLDL are utilized in carrying triglycerides from the liver into the bloodstream. The hydrolysis of triglycerides by lipoprotein lipase results in fat molecules being used by the body as energy or stored in fatty tissue. <ref name=”Arora”>PMID:31072929</ref><ref name=”Mead”>PMID:12483461</ref> | ||
==Structural Overview== | ==Structural Overview== | ||
| Line 12: | Line 14: | ||
=== GPIHBP1 === | === GPIHBP1 === | ||
| - | Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 ([ | + | Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 ([http://en.wikipedia.org/wiki/GPIHBP1 GPIHBP1]) is a secondary domain that is critical to the stabilization, function, and movement of <scene name='87/877513/Original_scene/1'>LPL</scene>.<ref name=”Birrane”>PMID:30559189</ref> The GPIHBP1’s highly acidic and intrinsically disordered N-terminal domain are essential to the binding of LPL’s C-terminal Domain. It has been shown that GPIHBP1 has a “three-fingered domain”, which holds it tightly to LPL by <scene name='87/877513/Hydrophobic_interface-labeled/3'>hydrophobic interactions</scene>.<ref name=”Birrane”>PMID:30559189</ref> The importance of GPIBP1’s affinity to LPL was analyzed by Birrane et al.<ref name=”Birrane”>PMID:30559189</ref>, and it was found that missense mutations of critical residues resulted in high amounts of impairments. It was also concluded that these impairments caused hypertriglyceridemia (chylomicronemia).<ref name=”Birrane”>PMID:30559189</ref> |
== Structural Highlights == | == Structural Highlights == | ||
| Line 20: | Line 22: | ||
=== Lid and Lipid Binding Region === | === Lid and Lipid Binding Region === | ||
In the presence of the GPIHBP1 inhibitor, the <scene name='87/877513/Lid_region_final/2'>lid region</scene> and <scene name='87/877516/Inhibitorsbound/1'>lipid-binding region</scene> become visible within the structure. As displayed through a study conducted by Arora et. al, in 2019, the lipid-binding region of LPL actively interacts with the known inhibitor in the heterodimeric form. <ref name=”Arora”>PMID:31072929</ref> This was established to be the only time that the heterodimeric form was shown as an active lipase. The lid region residues Ile245, Ile249, V251, Ile252, Leu257, Val260, Leu263, and Val264, are found as an open conformation which is composed of two small alpha helices that reach out and away from the protein. The lid and lipid-binding region create hydrophobic patches on the surface of lipoprotein lipase which are essential for <scene name='87/877513/Lipid_binding_and_lid/1'>ligand binding</scene> by LPL. | In the presence of the GPIHBP1 inhibitor, the <scene name='87/877513/Lid_region_final/2'>lid region</scene> and <scene name='87/877516/Inhibitorsbound/1'>lipid-binding region</scene> become visible within the structure. As displayed through a study conducted by Arora et. al, in 2019, the lipid-binding region of LPL actively interacts with the known inhibitor in the heterodimeric form. <ref name=”Arora”>PMID:31072929</ref> This was established to be the only time that the heterodimeric form was shown as an active lipase. The lid region residues Ile245, Ile249, V251, Ile252, Leu257, Val260, Leu263, and Val264, are found as an open conformation which is composed of two small alpha helices that reach out and away from the protein. The lid and lipid-binding region create hydrophobic patches on the surface of lipoprotein lipase which are essential for <scene name='87/877513/Lipid_binding_and_lid/1'>ligand binding</scene> by LPL. | ||
| - | + | [[Image:Inhibiting.png|300 px|right|thumb|The novel inhibitor bound between the lipid-binding region of one LPL and the catalytic site of the other LPL of the tetramer.]] | |
| - | + | ||
==Mechanism== | ==Mechanism== | ||
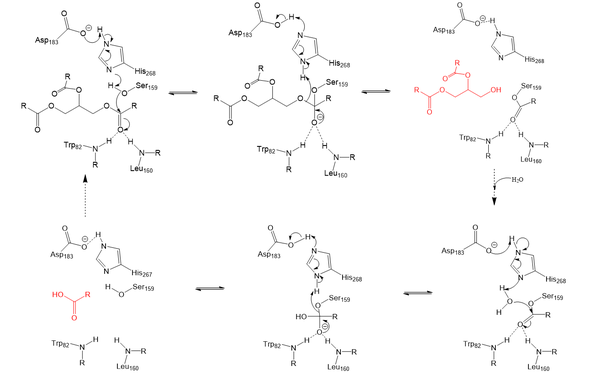
| - | Lipoprotein Lipase functions to catalyze the hydrolysis of one [ | + | Lipoprotein Lipase functions to catalyze the hydrolysis of one [http://en.wikipedia.org/wiki/Ester ester bond] of triglycerides in order to remove one fatty acid tail and turn the triglyceride into a diglyceride. It does this by utilizing a simple [http://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] mechanism, in which it uses a <scene name='87/877513/Catalytic_triad-1/6'>catalytic triad</scene> composed of Asp183, His268, and Ser159 to catalyze the hydrolysis. His268 serves as a base catalyst by deprotonation of Ser159, which can then serve as the [http://en.wikipedia.org/wiki/Nucleophile nucleophile] to attack the carbonyl carbon of one of the fatty acid chains of a triglyceride. This forms a tetrahedral intermediate, which is stabilized by the amide of Trp82 and Leu160 residues, called the <scene name='87/877513/Oxyanion_hole_-_labeled/9'>oxyanion hole</scene>. The single fatty acid chain is cleaved from the triglyceride, forming the diglyceride product. Then, water is used to free the fatty acid from Ser159 and return all of the residues back to their starting point, in order to perform the mechanism again on another triglyceride. Ultimately, the hydrolysis results in the formation of one free fatty acid and glycerol with two fatty acid tails (Figure 2). |
[[Image:LPL_final_Mechanism.png|600 px|center|thumb|Figure 2: Serine hydrolase mechanism utilized by LPL to catalyze the breakdown of one ester bond of a triglyceride. Compounds colored red are the products of the hydrolysis.]] | [[Image:LPL_final_Mechanism.png|600 px|center|thumb|Figure 2: Serine hydrolase mechanism utilized by LPL to catalyze the breakdown of one ester bond of a triglyceride. Compounds colored red are the products of the hydrolysis.]] | ||
== Relevance & Disease == | == Relevance & Disease == | ||
| - | LPL is an extremely important enzyme, in that it is responsible for the proper breakdown of certain fats in the body. LPL breaks down triglycerides carried in chylomicrons, otherwise known as very-low-density lipoproteins ([ | + | LPL is an extremely important enzyme, in that it is responsible for the proper breakdown of certain fats in the body. LPL breaks down triglycerides carried in chylomicrons, otherwise known as very-low-density lipoproteins ([http://en.wikipedia.org/wiki/Very_low-density_lipoprotein VLDL]). Chylomicrons carry digested fats in the form of triglycerides out of the small intestine and into the bloodstream. LPL recognizes the chylomicrons, and hydrolyzes the associated triglycerides.<ref name=”Kersten”>PMID:24721265</ref> When triglycerides are not broken down properly and are allowed to build up, they can lead to increased plasma triglyceride levels ([http://en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia]) and [http://en.wikipedia.org/wiki/Cholesterol cholesterol] buildup. Hypertriglyceridemia is very unhealthy and is the leading cause of [http://my.clevelandclinic.org/health/diseases/16898-coronary-artery-disease Coronary Artery Disease] in America.<ref name=”Austin”>PMID:9526807</ref> Cholesterol buildup is caused by excess fats (triglycerides) and is a similarly serious issue with regards to obesity and heart disease in the United States as it can lead to plaque buildup in arteries and veins, which restricts blood flow.<ref name=”Kruth”>PMID:11899253</ref> [http://medlineplus.gov/ency/article/000405.htm Chylomicronemia], which is defined as an excess of chylomicrons in the blood, is the disease characterized by the body being deficient in LPL resulting in persistent hypertriglyceridemia. This disease causes the body to be unable to digest very much ingested fats and often leads to severe abdominal discomfort and several episodes of acute pancreatitis.<ref name=”Francis”>PMID:11905095</ref> In short, without LPL in the body, triglycerides are unable to get broken down, and there is a much higher likelihood of developing coronary & metabolic based diseases. |
===M404 Mutation=== | ===M404 Mutation=== | ||
| - | A 2018 study <ref name=”Paquette”>PMID:29452893</ref> argued that mutations that inhibit the proper binding of GPIHBP1 and LPL are suspected to cause chylomicronemia. A mutation that is believed to cause chylomicronemia is known as <scene name='87/ | + | A 2018 study <ref name=”Paquette”>PMID:29452893</ref> argued that mutations that inhibit the proper binding of GPIHBP1 and LPL are suspected to cause chylomicronemia. A mutation that is believed to cause chylomicronemia is known as <scene name='87/877513/M404_mutation_site/2'>M404R</scene>. Within this mutation, the methionine is replaced with a large and hydrophilic arginine. While this missense mutation does not impact LPL secretion, it does affect the formation of the LPL-GPIHBP1 complex by disrupting LPL’s interaction with GPIHB1’s Val121, Glu122, Thr124, and Val126 residues. This disrupts the stabilization of the LPL-GPIHBP1 complex, which has been seen to negatively affect LPL’s ability to catalyze the hydrolysis of triglycerides. |
Revision as of 02:33, 28 April 2021
Lipoprotein Lipase coupled with GPIHBP1
| |||||||||||