This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Firefly Luciferase== | ==Firefly Luciferase== | ||
| - | + | losing my mind B~) | |
<StructureSection loadfiles='4G36''4G37' size='340' side='right' caption='Luciferin-4-monooxygenase. The wild-type luciferase in the adenylate-forming conformation with DLSA (PDB 4G36) and the cross-linked luciferase in the second catalytic conformation with DLSA (PDB 4G37)' scene=''> | <StructureSection loadfiles='4G36''4G37' size='340' side='right' caption='Luciferin-4-monooxygenase. The wild-type luciferase in the adenylate-forming conformation with DLSA (PDB 4G36) and the cross-linked luciferase in the second catalytic conformation with DLSA (PDB 4G37)' scene=''> | ||
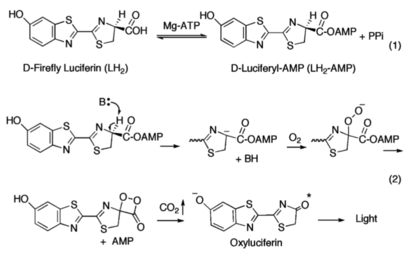
Firefly luciferase, of the common eastern firefly (''Photinus pyralis''), is responsible for the ability of the firefly to exhibit bioluminescence. The enzyme luciferin-4-monoxygenase, which catalyzes a multistep oxidative decarboxylation of the luciferyl-AMP intermediate (LH<sub>2</sub>-AMP) to produce bioluminescence, is a part of the ANL superfamily named so after the '''a'''cyl-CoA syntheses, the adenylation domains of the modular '''n'''on-ribosomal peptide synthetases (NRPs), and '''l'''uciferase. | Firefly luciferase, of the common eastern firefly (''Photinus pyralis''), is responsible for the ability of the firefly to exhibit bioluminescence. The enzyme luciferin-4-monoxygenase, which catalyzes a multistep oxidative decarboxylation of the luciferyl-AMP intermediate (LH<sub>2</sub>-AMP) to produce bioluminescence, is a part of the ANL superfamily named so after the '''a'''cyl-CoA syntheses, the adenylation domains of the modular '''n'''on-ribosomal peptide synthetases (NRPs), and '''l'''uciferase. | ||
| Line 8: | Line 8: | ||
The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | The ANL enzymes catalyze two-step reactions: the first an adenylating step in which an acyl-AMP intermediate is produced; the second step in which the adenylate then serves as a substrate for the multistep oxidative decarboxylation of the luciferyl-AMP (LH<sub>2</sub>-AMP) intermediate, resulting in bioluminescence. | ||
| - | ANL enzymes follow a domain alternation strategy for the first adenylation reaction, in which the reaction is catalyzed by <scene name='75/752266/4g36/2'>one conformation</scene> and following the formation of the adenylate intermediate and release of pyrophosphate (PPi), the C-terminal domain undergoes a rotational transformation that is necessary for <scene name='75/752266/4g37/2'>the second partial reaction</scene>. The <scene name='75/752266/Active_site/1'>active site</scene><ref name=“Branchini”>Branchini, B. R., Magyar, R. A., Murtiashaw, M. H., Anderson, S. M., Helgerson, L. C., & Zimmer, M. (1999). Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color. ''Biochemistry 38''(40), 13223–13230. https://doi.org/10.1021/bi991181o</ref> of ANL enzymes resides between a 400-500 residue N-terminal domain and a smaller C-terminal domain of ~110-130 amino acids<ref name="Sundlov">Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. ''Biochemistry 51''(33), 6493-6495. https://doi.org/10.1021/bi300934s</ref>. Ten conserved regions of these proteins have been termed the A1-A10 motifs which play critical roles in either or both partial reactions<ref name="Marahiel">Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. ''Chemical Reviews 97''(7), 2651-2674. https://doi.org/10.1021/cr960029e</ref>. Two lysine residues are required for each partial reaction, suggestive that luciferase similarly adopts a rotational transformation for complete catalysis. A mutation of <scene name='75/752266/Lys529/1'>Lys529</scene>, the A10 lysine, impairs only the initial adenylation reaction<ref name="Sundlov"/> whereas mutation of <scene name='75/752266/Lys443/1'>Lys443</scene> in the A8 region disrupts the oxidative reaction<ref name="Sundlov"/>. | + | ANL enzymes follow a domain alternation strategy for the first adenylation reaction, in which the reaction is catalyzed by <scene name='75/752266/4g36/2'>one conformation</scene>, and following the formation of the adenylate intermediate and release of pyrophosphate (PPi), the C-terminal domain undergoes a rotational transformation that is necessary for <scene name='75/752266/4g37/2'>the second partial reaction</scene>. The <scene name='75/752266/Active_site/1'>active site</scene><ref name=“Branchini”>Branchini, B. R., Magyar, R. A., Murtiashaw, M. H., Anderson, S. M., Helgerson, L. C., & Zimmer, M. (1999). Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color. ''Biochemistry 38''(40), 13223–13230. https://doi.org/10.1021/bi991181o</ref> of ANL enzymes resides between a 400-500 residue N-terminal domain and a smaller C-terminal domain of ~110-130 amino acids<ref name="Sundlov">Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. ''Biochemistry 51''(33), 6493-6495. https://doi.org/10.1021/bi300934s</ref>. Ten conserved regions of these proteins have been termed the A1-A10 motifs which play critical roles in either or both partial reactions<ref name="Marahiel">Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. ''Chemical Reviews 97''(7), 2651-2674. https://doi.org/10.1021/cr960029e</ref>. Two lysine residues are required for each partial reaction, suggestive that luciferase similarly adopts a rotational transformation for complete catalysis. A mutation of <scene name='75/752266/Lys529/1'>Lys529</scene>, the A10 lysine, impairs only the initial adenylation reaction<ref name="Sundlov"/> whereas mutation of <scene name='75/752266/Lys443/1'>Lys443</scene> in the A8 region disrupts the oxidative reaction<ref name="Sundlov"/>. |
=== Biochemical Mechanism of LH2-AMP Oxidation=== | === Biochemical Mechanism of LH2-AMP Oxidation=== | ||
Revision as of 12:59, 28 April 2021
Firefly Luciferase
losing my mind B~)
| |||||||||||
References
- ↑ Branchini, B. R., Magyar, R. A., Murtiashaw, M. H., Anderson, S. M., Helgerson, L. C., & Zimmer, M. (1999). Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color. Biochemistry 38(40), 13223–13230. https://doi.org/10.1021/bi991181o
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. Biochemistry 51(33), 6493-6495. https://doi.org/10.1021/bi300934s
- ↑ Marahiel, M. A., Stachelhaus, T., Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. Chemical Reviews 97(7), 2651-2674. https://doi.org/10.1021/cr960029e
- ↑ 4.0 4.1 Branchini, B. R., Southworth, T. L., Murtiahsaw, M. H., Wilkinson, S. R., Khattak, N. F., Rosenberg, J. C., & Zimmer, M. (2005). Mutagenesis Evidence that the Partial Reactions of Firefly Bioluminescence are Catalyzed by Different Conformations of the Luciferase C-Terminal Domain. “Biochemistry 44”(5), 1385-1393. https://doi.org/10.1021/bi047903f
- ↑ Oba, Y., Ojika, M., Inouye, S. (2003). Firefly luciferase is a bifunctional enzyme: ATP-dependent monoxygenase and a long chain fatty acyl-CoA synthetase. “FEBS Letters 540”(1-3), 251-254. https://doi.org/10.1016/S0014-5793(03)00272-2
- ↑ Nakamura, M., Maki, S., Amano, Y., Ohkita, Y., Niwa, K., Hirano, T., Ohmiya, Y., & Niwa, H. (2005). Firefly luciferase exhibits bimodal action depending on the luciferin chirality. “Biochemical and Biophysical Research Communications, 331”(2), 471–475. https://doi.org/10.1016/j.bbrc.2005.03.202
- ↑ Branchini, B. R., Murtiashaw, M. H., Magyar, R. A., Anderson, S. M. (2000). The Role of Lysine 529, a Conserved Residue of the Acyl-Adenylate-Forming Enzyme Superfamily, in Firefly Luciferase. Biochemistry 39(18), 5433-5440. https://doi.org/10.1021/bi9928804
- ↑ Sala-Newby, G. B., & Campbell, A. K. (1991). Engineering a bioluminescent indicator for cyclic AMP-dependent protein kinase. “The Biochemical Journal”, 279 (Pt 3), 727–732. https://doi.org/10.1042/bj2790727
- ↑ de Wet, J. R., Wood, K. V., DeLuca, M., Helinski, D. R., & Subramani, S. (1987). Firefly luciferase gene: structure and expression in mammalian cells. Molecular and cellular biology, 7(2), 725–737. https://doi.org/10.1128/mcb.7.2.725
- ↑ de Wet, J. R., Wood, K. V., Helinski, D. R., & DeLuca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 82(23), 7870–7873. https://doi.org/10.1073/pnas.82.23.7870
- ↑ Thorne, N., Shen, M., Lea, W. A., Simeonov, A., Lovell, S., Auld, D. S., & Inglese, J. (2012). Firefly luciferase in chemical biology: a compendium of inhibitors, mechanistic evaluation of chemotypes, and suggested use as a reporter. Chemistry & biology, 19(8), 1060–1072. https://doi.org/10.1016/j.chembiol.2012.07.015
![The Common Eastern Firefly in a hand emitting a yellow hue, showing bioluminescence.[1]](/wiki/images/thumb/6/62/Common_Eastern_Firefly.jpg/200px-Common_Eastern_Firefly.jpg)
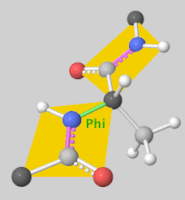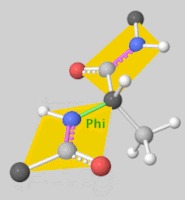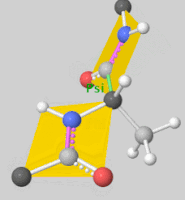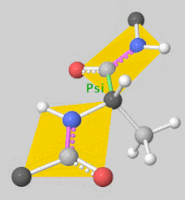
![5'-O-[N-(Dehydroluciferyl)-sulfamoyl] adenosine, shortened to DLSA for brevity. The sulfamate moiety is shown to the far left (sulfur atoms are represented in yellow while oxygen atoms are represented in red). Further, the carbonyl oxygen of the luciferyl-adenylate is connected to the sulfamate moiety via nitrogen atom (represented in blue). [2]](/wiki/images/thumb/6/61/DLSA.png/410px-DLSA.png)
![The Common Eastern Firefly expressing bioluminescence seen giving off a yellow-green hue.[3]](/wiki/images/thumb/e/e9/CommonEasternFirefly.jpg/320px-CommonEasternFirefly.jpg)
