We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC3
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Firefly Luciferase== | ==Firefly Luciferase== | ||
| - | + | ||
<StructureSection loadfiles='4G36''4G37' size='340' side='right' caption='Luciferin-4-monooxygenase. The wild-type luciferase in the adenylate-forming conformation with DLSA (PDB 4G36) and the cross-linked luciferase in the second catalytic conformation with DLSA (PDB 4G37)' scene=''> | <StructureSection loadfiles='4G36''4G37' size='340' side='right' caption='Luciferin-4-monooxygenase. The wild-type luciferase in the adenylate-forming conformation with DLSA (PDB 4G36) and the cross-linked luciferase in the second catalytic conformation with DLSA (PDB 4G37)' scene=''> | ||
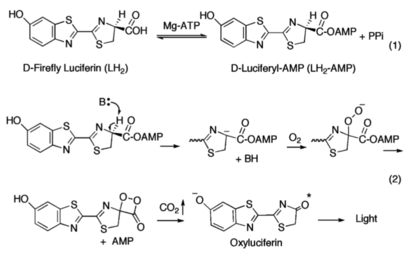
Firefly luciferase, of the common eastern firefly (''Photinus pyralis''), is responsible for the ability of the firefly to exhibit bioluminescence. The enzyme luciferin-4-monoxygenase, which catalyzes a multistep oxidative decarboxylation of the luciferyl-AMP intermediate (LH<sub>2</sub>-AMP) to produce bioluminescence, is a part of the ANL superfamily named so after the '''a'''cyl-CoA syntheses, the adenylation domains of the modular '''n'''on-ribosomal peptide synthetases (NRPs), and '''l'''uciferase. | Firefly luciferase, of the common eastern firefly (''Photinus pyralis''), is responsible for the ability of the firefly to exhibit bioluminescence. The enzyme luciferin-4-monoxygenase, which catalyzes a multistep oxidative decarboxylation of the luciferyl-AMP intermediate (LH<sub>2</sub>-AMP) to produce bioluminescence, is a part of the ANL superfamily named so after the '''a'''cyl-CoA syntheses, the adenylation domains of the modular '''n'''on-ribosomal peptide synthetases (NRPs), and '''l'''uciferase. | ||
| Line 17: | Line 17: | ||
The first partial reaction entails the conversion of the carboxyl group of <small>D</small>-luciferin<ref name="Sundlov"/><ref name="Bruce">Branchini, B. R., Southworth, T. L., Murtiahsaw, M. H., Wilkinson, S. R., Khattak, N. F., Rosenberg, J. C., & Zimmer, M. (2005). Mutagenesis Evidence that the Partial Reactions of Firefly Bioluminescence are Catalyzed by Different Conformations of the Luciferase C-Terminal Domain. “Biochemistry 44”(5), 1385-1393. https://doi.org/10.1021/bi047903f</ref><ref name="Nakamura">Nakamura, M., Maki, S., Amano, Y., Ohkita, Y., Niwa, K., Hirano, T., Ohmiya, Y., & Niwa, H. (2005). Firefly luciferase exhibits bimodal action depending on the luciferin chirality. “Biochemical and Biophysical Research Communications, 331”(2), 471–475. https://doi.org/10.1016/j.bbrc.2005.03.202</ref> by luciferase in the presence of ATP and Mg<sup>2+</sup>, yielding luciferyl-adenylate (LH<sub>2</sub>-AMP) and pyrophosphate as a by-product. Amino acid residues subsequently are recruited to promote the oxidation of LH<sub>2</sub>-AMP using molecular oxygen by luciferase (acting as a monooxygenase)<ref name="Oba">Oba, Y., Ojika, M., & Inouye, S. (2003). Firefly luciferase is a bifunctional enzyme: ATP-dependent monoxygenase and a long chain fatty acyl-CoA synthetase. “FEBS Letters 540”(1-3), 251-254. https://doi.org/10.1016/S0014-5793(03)00272-2</ref>, which then eventually yields oxyluciferin in the excited-state and CO<sub>2</sub>. It is upon the return from the excited-state to the ground state that the emittance of a yellow-green light is observed (λ≈560 nm)<ref name="Nakamura"/>. | The first partial reaction entails the conversion of the carboxyl group of <small>D</small>-luciferin<ref name="Sundlov"/><ref name="Bruce">Branchini, B. R., Southworth, T. L., Murtiahsaw, M. H., Wilkinson, S. R., Khattak, N. F., Rosenberg, J. C., & Zimmer, M. (2005). Mutagenesis Evidence that the Partial Reactions of Firefly Bioluminescence are Catalyzed by Different Conformations of the Luciferase C-Terminal Domain. “Biochemistry 44”(5), 1385-1393. https://doi.org/10.1021/bi047903f</ref><ref name="Nakamura">Nakamura, M., Maki, S., Amano, Y., Ohkita, Y., Niwa, K., Hirano, T., Ohmiya, Y., & Niwa, H. (2005). Firefly luciferase exhibits bimodal action depending on the luciferin chirality. “Biochemical and Biophysical Research Communications, 331”(2), 471–475. https://doi.org/10.1016/j.bbrc.2005.03.202</ref> by luciferase in the presence of ATP and Mg<sup>2+</sup>, yielding luciferyl-adenylate (LH<sub>2</sub>-AMP) and pyrophosphate as a by-product. Amino acid residues subsequently are recruited to promote the oxidation of LH<sub>2</sub>-AMP using molecular oxygen by luciferase (acting as a monooxygenase)<ref name="Oba">Oba, Y., Ojika, M., & Inouye, S. (2003). Firefly luciferase is a bifunctional enzyme: ATP-dependent monoxygenase and a long chain fatty acyl-CoA synthetase. “FEBS Letters 540”(1-3), 251-254. https://doi.org/10.1016/S0014-5793(03)00272-2</ref>, which then eventually yields oxyluciferin in the excited-state and CO<sub>2</sub>. It is upon the return from the excited-state to the ground state that the emittance of a yellow-green light is observed (λ≈560 nm)<ref name="Nakamura"/>. | ||
| - | An alternative mechanism involving the enantiomer of <small>D</small>-luciferin exists, though typically <small>L</small>-luciferin acts as a competitive inhibitor to the bioluminescence-producing reaction<ref name=“Seliger”>Seliger, H. H., McElroy, W. D., White, E. H., & Field, G. F. (1961). Stereospecificity and firefly bioluminescence, a comparison of natural and synthetic luciferins. ‘’Proceedings of the National Academy of Sciences of the United States of America 47’’(8), 1129-1134. https://doi.org/10.1073/pnas.47.8.1129</ref>, though accounts of light production in small quantities have previously been reported<ref name=“Lembert”>Lembert, N. (1996). Firefly luciferase can use L-luciferin to produce light. ‘’Biochemical Journal 317’’(1), 273-277. https://doi.org/10.1042/bj3170273</ref>. The mechanism by which L-luciferin acts as the substrate in the presence of luciferase (and ATP and Mg<sup>2+</sup>) is the same in the first partial reaction, with both producing the intermediate luciferyl-adenylate. Rather than the oxidative decarboxylation step, the adenyl group (AMP) is substituted with CoA-SH yielding luciferyl-CoA. Furthermore, the stereospecificity of luciferase has shown that even in the presence of CoA-SH, <small>D</small>-luciferin was not converted into luciferyl-CoA but proceeded in being used for the emittance of light<ref name="Nakamura"/>. | + | An alternative mechanism involving the enantiomer of <small>D</small>-luciferin exists, though typically <small>L</small>-luciferin acts as a competitive inhibitor to the bioluminescence-producing reaction<ref name=“Seliger”>Seliger, H. H., McElroy, W. D., White, E. H., & Field, G. F. (1961). Stereospecificity and firefly bioluminescence, a comparison of natural and synthetic luciferins. ‘’Proceedings of the National Academy of Sciences of the United States of America 47’’(8), 1129-1134. https://doi.org/10.1073/pnas.47.8.1129</ref>, though accounts of light production in small quantities have previously been reported<ref name=“Lembert”>Lembert, N. (1996). Firefly luciferase can use L-luciferin to produce light. ‘’Biochemical Journal 317’’(1), 273-277. https://doi.org/10.1042/bj3170273</ref>. The mechanism by which <small>L</small>-luciferin acts as the substrate in the presence of luciferase (and ATP and Mg<sup>2+</sup>) is the same in the first partial reaction, with both producing the intermediate luciferyl-adenylate. Rather than the oxidative decarboxylation step, the adenyl group (AMP) is substituted with CoA-SH yielding luciferyl-CoA. Furthermore, the stereospecificity of luciferase has shown that even in the presence of CoA-SH, <small>D</small>-luciferin was not converted into luciferyl-CoA but proceeded in being used for the emittance of light<ref name="Nakamura"/>. |
| - | + | ||
Current revision
Firefly Luciferase
| |||||||||||
References
- ↑ Branchini, B. R., Magyar, R. A., Murtiashaw, M. H., Anderson, S. M., Helgerson, L. C., & Zimmer, M. (1999). Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color. Biochemistry 38(40), 13223–13230. https://doi.org/10.1021/bi991181o
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R., & Gulick, A. M. (2012). Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. Biochemistry 51(33), 6493-6495. https://doi.org/10.1021/bi300934s
- ↑ Marahiel, M. A., Stachelhaus, T., & Mootz, H. D. (1997). Modular Peptide Synthetases Involved in Nonribosmal Peptide Synthesis. Chemical Reviews 97(7), 2651-2674. https://doi.org/10.1021/cr960029e
- ↑ 4.0 4.1 Branchini, B. R., Southworth, T. L., Murtiahsaw, M. H., Wilkinson, S. R., Khattak, N. F., Rosenberg, J. C., & Zimmer, M. (2005). Mutagenesis Evidence that the Partial Reactions of Firefly Bioluminescence are Catalyzed by Different Conformations of the Luciferase C-Terminal Domain. “Biochemistry 44”(5), 1385-1393. https://doi.org/10.1021/bi047903f
- ↑ 5.0 5.1 5.2 Nakamura, M., Maki, S., Amano, Y., Ohkita, Y., Niwa, K., Hirano, T., Ohmiya, Y., & Niwa, H. (2005). Firefly luciferase exhibits bimodal action depending on the luciferin chirality. “Biochemical and Biophysical Research Communications, 331”(2), 471–475. https://doi.org/10.1016/j.bbrc.2005.03.202
- ↑ Oba, Y., Ojika, M., & Inouye, S. (2003). Firefly luciferase is a bifunctional enzyme: ATP-dependent monoxygenase and a long chain fatty acyl-CoA synthetase. “FEBS Letters 540”(1-3), 251-254. https://doi.org/10.1016/S0014-5793(03)00272-2
- ↑ Seliger, H. H., McElroy, W. D., White, E. H., & Field, G. F. (1961). Stereospecificity and firefly bioluminescence, a comparison of natural and synthetic luciferins. ‘’Proceedings of the National Academy of Sciences of the United States of America 47’’(8), 1129-1134. https://doi.org/10.1073/pnas.47.8.1129
- ↑ Lembert, N. (1996). Firefly luciferase can use L-luciferin to produce light. ‘’Biochemical Journal 317’’(1), 273-277. https://doi.org/10.1042/bj3170273
- ↑ Branchini, B. R., Murtiashaw, M. H., Magyar, R. A., Anderson, S. M. (2000). The Role of Lysine 529, a Conserved Residue of the Acyl-Adenylate-Forming Enzyme Superfamily, in Firefly Luciferase. Biochemistry 39(18), 5433-5440. https://doi.org/10.1021/bi9928804
- ↑ Sala-Newby, G. B., & Campbell, A. K. (1991). Engineering a bioluminescent indicator for cyclic AMP-dependent protein kinase. “The Biochemical Journal”, 279 (Pt 3), 727–732. https://doi.org/10.1042/bj2790727
- ↑ de Wet, J. R., Wood, K. V., DeLuca, M., Helinski, D. R., & Subramani, S. (1987). Firefly luciferase gene: structure and expression in mammalian cells. Molecular and cellular biology, 7(2), 725–737. https://doi.org/10.1128/mcb.7.2.725
- ↑ de Wet, J. R., Wood, K. V., Helinski, D. R., & DeLuca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 82(23), 7870–7873. https://doi.org/10.1073/pnas.82.23.7870
- ↑ Thorne, N., Shen, M., Lea, W. A., Simeonov, A., Lovell, S., Auld, D. S., & Inglese, J. (2012). Firefly luciferase in chemical biology: a compendium of inhibitors, mechanistic evaluation of chemotypes, and suggested use as a reporter. Chemistry & biology, 19(8), 1060–1072. https://doi.org/10.1016/j.chembiol.2012.07.015
![The Common Eastern Firefly in a hand emitting a yellow hue, showing bioluminescence.[1]](/wiki/images/thumb/6/62/Common_Eastern_Firefly.jpg/200px-Common_Eastern_Firefly.jpg)
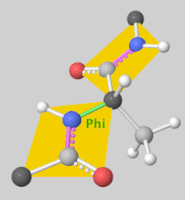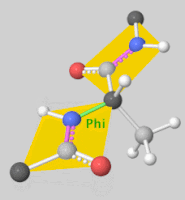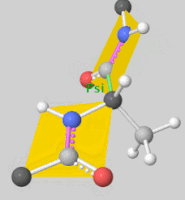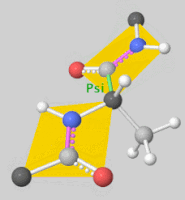
![5'-O-[N-(Dehydroluciferyl)-sulfamoyl] adenosine, shortened to DLSA for brevity. The sulfamate moiety is shown to the far left (sulfur atoms are represented in yellow while oxygen atoms are represented in red). Further, the carbonyl oxygen of the luciferyl-adenylate is connected to the sulfamate moiety via nitrogen atom (represented in blue). [2]](/wiki/images/thumb/6/61/DLSA.png/410px-DLSA.png)
![The Common Eastern Firefly expressing bioluminescence seen giving off a yellow-green hue.[3]](/wiki/images/thumb/e/e9/CommonEasternFirefly.jpg/320px-CommonEasternFirefly.jpg)
