Intracellular receptors
From Proteopedia
(Difference between revisions)
| Line 96: | Line 96: | ||
* [[Tamoxifen|Tamoxifen and the Estrogen receptor]] | * [[Tamoxifen|Tamoxifen and the Estrogen receptor]] | ||
* [[Student Project 10 for UMass Chemistry 423 Spring 2015]] | * [[Student Project 10 for UMass Chemistry 423 Spring 2015]] | ||
| - | <scene name='48/483891/Initial_view/1'>Estrogen receptor β</scene> (ER-β) is | + | <scene name='48/483891/Initial_view/1'>Estrogen receptor β</scene> (ER-β) is 1 of the 2 isoforms of the estrogen receptor, a ligand-activated transcription factor which regulates the biological effects of the steroid hormone 17 β-estradiol, or estrogen, in both males and females. The complex is a hetero-tetrameric assembly consisting of 4 molecules and a ligand: 2 copies of <scene name='48/483891/Erbeta/1'>estrogen receptor β</scene>, 2 copies of <scene name='48/483891/Steroid_receptor/3'>steroid receptor coactivator-1</scene>, and the ligand, <scene name='48/483891/Ligand/3'>Genistein</scene>. Once the ligand is bound, the complex recruits the steroid receptor coactivators, which recruit other proteins to form the transcriptional complex for initiation of transcription. This activates expression of reporter genes containing estrogen response elements. Genistein is a phytoestrogen with structural similarity to estrogen which competes for estrogen receptors. |
Although estrogen receptor β is widely expressed, it is not the primary estrogen receptor in most tissues. As a result, it has become a target for drug delivery, especially since it is 40x more selective for genistein than the α isoform. This enhanced selectivity may be caused by differences in residues <scene name='48/483891/Met336_ile373/2'>336 and 373</scene> between the 2 isoforms, allowing ER-β to accommodate more polar substituents in its binding pocket. ER-β differs greatly from ER-α at the N-terminal domains, which can be seen located at opposite ends from the C termini in this <scene name='48/483891/Rainbow/1'>rainbow representation</scene>. The protein is composed of 3 sections: a modulating N-terminal domain, a DNA-binding domain and a C-terminal ligand-binding domain. | Although estrogen receptor β is widely expressed, it is not the primary estrogen receptor in most tissues. As a result, it has become a target for drug delivery, especially since it is 40x more selective for genistein than the α isoform. This enhanced selectivity may be caused by differences in residues <scene name='48/483891/Met336_ile373/2'>336 and 373</scene> between the 2 isoforms, allowing ER-β to accommodate more polar substituents in its binding pocket. ER-β differs greatly from ER-α at the N-terminal domains, which can be seen located at opposite ends from the C termini in this <scene name='48/483891/Rainbow/1'>rainbow representation</scene>. The protein is composed of 3 sections: a modulating N-terminal domain, a DNA-binding domain and a C-terminal ligand-binding domain. | ||
{{Template:ColorKey_N2CRainbow}} | {{Template:ColorKey_N2CRainbow}} | ||
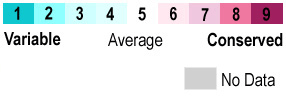
| - | Each ERβ contains several domains with specific functions: an N-terminal domain (NTD), a {{Template:ColorKey Composition DNA}}-binding domain (DBD), a flexible hinge region and a C-terminal {{Template:ColorKey Composition Ligand}}-binding domain (LBD). The complex overall is about <scene name='48/483891/Erhelices/1'>66% helical (10 helices; 160 residues) and 3% β-sheet (2 strands; 9 residues)</scene>. | + | Each ERβ contains several domains with specific functions: an N-terminal domain (NTD), a {{Template:ColorKey Composition DNA}}-binding domain (DBD), a flexible hinge region and a C-terminal {{Template:ColorKey Composition Ligand}}-binding domain (LBD). The complex overall is about <scene name='48/483891/Erhelices/1'>66% helical (10 helices; 160 residues) and 3% β-sheet (2 strands; 9 residues)</scene>. The <scene name='48/483891/Ntd-erbeta/2'>NTD</scene> is the 1st activation function (AF-1) domain that consists mostly of random coils and a small portion of helices (red) and sheets (green); it is a <scene name='48/483891/Sequence_conservation/1'>variable region</scene>. This lack of structure allows the region to recruit and bond many different interaction partners. This region also has the capacity to transactivate transcription without binding estrogen. |
| - | + | ||
| - | The <scene name='48/483891/Ntd-erbeta/2'>NTD</scene> is the 1st activation function (AF-1) domain that consists mostly of random coils and a small portion of helices (red) and sheets (green); it is a <scene name='48/483891/Sequence_conservation/1'>variable region</scene>. This lack of structure allows the region to recruit and bond many different interaction partners. This region also has the capacity to transactivate transcription without binding estrogen. | + | |
{{Template:ColorKey_ConSurf_NoYellow}} | {{Template:ColorKey_ConSurf_NoYellow}} | ||
| Line 116: | Line 114: | ||
Binding at the LBD activates transcription mediated by the DBD. This domain is entirely helical; the LBD interacts with genistein through helices. The conformationally dynamic portion of this region gives rise to ERβ’s ligand-dependent transcriptional activation (AF-2) function. A key element of AF-2 is helix 12 (H12), which acts as a conformational switch; different receptor ligands influence the orientation of H12. Agonist ligands like genistein position H12 across the ligand-binding pocket of the LBD, which provides a coactivator docking surface. Geinstein binding allows the helices of AF-2 to form a shallow hydrophobic binding site for leucine-rich motifs of coactivators to bind. This conformation provides optimal interaction with coactivators and transcription is activated. | Binding at the LBD activates transcription mediated by the DBD. This domain is entirely helical; the LBD interacts with genistein through helices. The conformationally dynamic portion of this region gives rise to ERβ’s ligand-dependent transcriptional activation (AF-2) function. A key element of AF-2 is helix 12 (H12), which acts as a conformational switch; different receptor ligands influence the orientation of H12. Agonist ligands like genistein position H12 across the ligand-binding pocket of the LBD, which provides a coactivator docking surface. Geinstein binding allows the helices of AF-2 to form a shallow hydrophobic binding site for leucine-rich motifs of coactivators to bind. This conformation provides optimal interaction with coactivators and transcription is activated. | ||
| - | Genistein's bicyclic form allows it to hydrogen bond on opposite sides with the hydroxyls of the histidine groups on the receptor. <scene name='48/483891/Estrogen_kyle/12'>His475's</scene> binding to the receptor causes a conformational change and activates the receptor resulting in up-regulation for coactivators. Down-regulation will occur in the presence of corepressor as they bind to repressors and indirectly regulate gene expression. | + | Genistein's bicyclic form allows it to hydrogen bond on opposite sides with the hydroxyls of the histidine groups on the receptor. <scene name='48/483891/Estrogen_kyle/12'>His475's</scene> binding to the receptor causes a conformational change and activates the receptor resulting in up-regulation for coactivators. Down-regulation will occur in the presence of corepressor as they bind to repressors and indirectly regulate gene expression. In order for the estrogen receptor β genistein to bind to a receptor and activate it there must be stabilization by a coactivator. The coactivator increases the gene expression and with this increase allows it to bind to an activator group consisting of a DNA binding domain. The estrogen receptor is found to be comprised of a dimer attached to a ligand and coactivator peptide which helps to stabilize the structure of each monomer. The conformational state of helix-12 can be modified by the binding of the coactivator. |
This <scene name='48/483891/Estrogen_kyle/8'>scene</scene> depicts the hydrophobic and hydrophilic residues of the estrogen receptor. The hydrophobic regions are primarily on the inside of the protein surrounding genistein (red). Having the hydrophobic residues surrounding the binding pocket will stabilize the structure. The structure of this pocket is tertiary and do to the hydrophobic interactions inside the pocket and hydrophilic interactions on the outside help to stabilize this tertiary structure. The <scene name='48/483891/Estrogen_kyle/16'>binding pocket</scene> is hydrophobic which means that an increase in lipophilicity would increase the affinity for ligands which in this case is genistein. The genistein structure has 3 hydroxyl groups, an ether and an ester. These 3 functional groups are polar and have many possibilities for hydrogen bonding. The His475 and Met336 residues in the binding pocket are capable of forming hydrogen bonds with genistein do to the many hydrogen bond forming functional groups. These residues are different from the residues found in ERα and so the selectivity of genistein is much greater for ERβ. | This <scene name='48/483891/Estrogen_kyle/8'>scene</scene> depicts the hydrophobic and hydrophilic residues of the estrogen receptor. The hydrophobic regions are primarily on the inside of the protein surrounding genistein (red). Having the hydrophobic residues surrounding the binding pocket will stabilize the structure. The structure of this pocket is tertiary and do to the hydrophobic interactions inside the pocket and hydrophilic interactions on the outside help to stabilize this tertiary structure. The <scene name='48/483891/Estrogen_kyle/16'>binding pocket</scene> is hydrophobic which means that an increase in lipophilicity would increase the affinity for ligands which in this case is genistein. The genistein structure has 3 hydroxyl groups, an ether and an ester. These 3 functional groups are polar and have many possibilities for hydrogen bonding. The His475 and Met336 residues in the binding pocket are capable of forming hydrogen bonds with genistein do to the many hydrogen bond forming functional groups. These residues are different from the residues found in ERα and so the selectivity of genistein is much greater for ERβ. | ||
| - | + | Upon visualizing the estrogen receptor in an <scene name='48/483891/Arrow_view/1'>arrow representation</scene>, the structure can be classified as parallel or anti-parallel. Here is the zoomed <scene name='48/483891/Hydrophobic_pocket/3'>primarily hydrophobic pocket</scene>. | |
*[[Estrogen-related receptor]] | *[[Estrogen-related receptor]] | ||
<scene name='50/501401/Cv/4'>Binding of nuclear receptor corepressor 2 peptide and 4-hydroxytamoxifen</scene> to human estrogen-related receptor γ. The chemotherapeutic drugs bisphenol and <scene name='50/501401/Cv/5'>tamoxifen</scene> are nestled between 4 alpha helices in the ERR active site. | <scene name='50/501401/Cv/4'>Binding of nuclear receptor corepressor 2 peptide and 4-hydroxytamoxifen</scene> to human estrogen-related receptor γ. The chemotherapeutic drugs bisphenol and <scene name='50/501401/Cv/5'>tamoxifen</scene> are nestled between 4 alpha helices in the ERR active site. | ||
Revision as of 13:27, 23 May 2021
| |||||||||||
References
- ↑ Li MJ, Greenblatt HM, Dym O, Albeck S, Pais A, Gunanathan C, Milstein D, Degani H, Sussman JL. Structure of estradiol metal chelate and estrogen receptor complex: The basis for designing a new class of selective estrogen receptor modulators. J Med Chem. 2011 Apr 7. PMID:21473635 doi:10.1021/jm200192y
- ↑ Bledsoe RK, Madauss KP, Holt JA, Apolito CJ, Lambert MH, Pearce KH, Stanley TB, Stewart EL, Trump RP, Willson TM, Williams SP. A ligand-mediated hydrogen bond network required for the activation of the mineralocorticoid receptor. J Biol Chem. 2005 Sep 2;280(35):31283-93. Epub 2005 Jun 20. PMID:15967794 doi:http://dx.doi.org/10.1074/jbc.M504098200