DOPA decarboxylase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
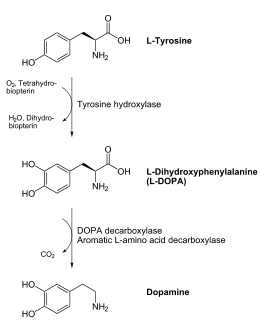
[[image:dopa.png|thumb|left|300px|'''Dopamine Synthesis''']] | [[image:dopa.png|thumb|left|300px|'''Dopamine Synthesis''']] | ||
{{Clear}} | {{Clear}} | ||
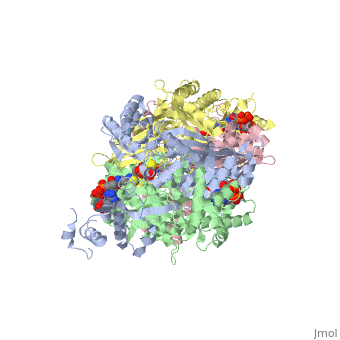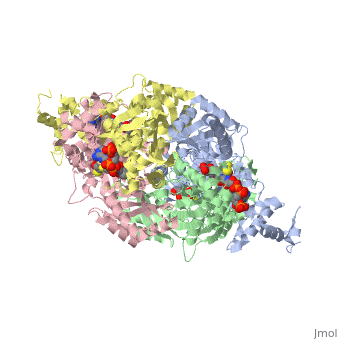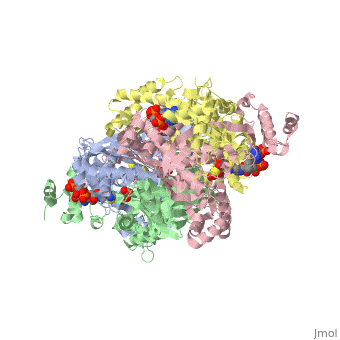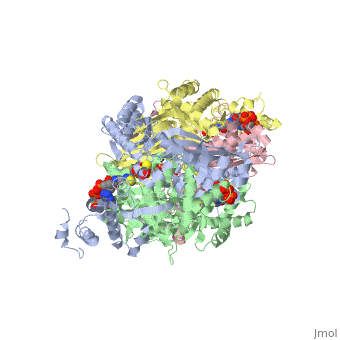
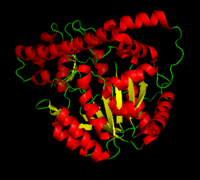
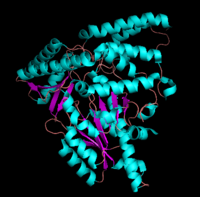
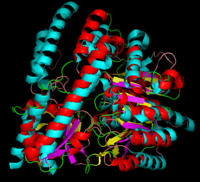
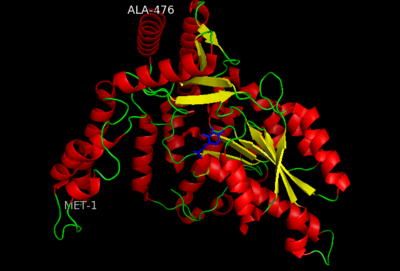
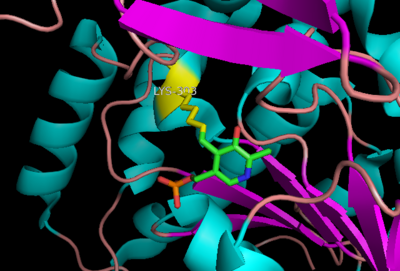
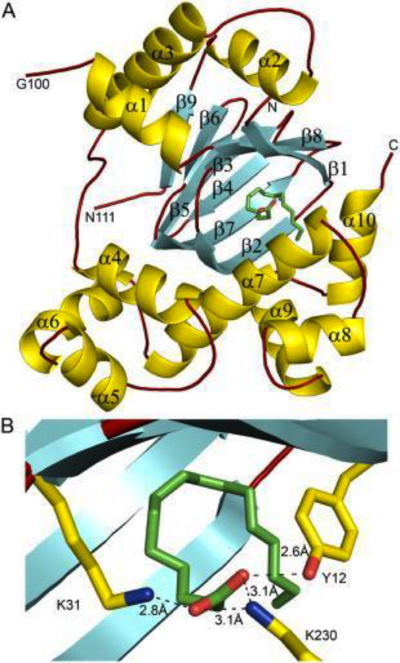
| - | '''DOPA decarboxylase''' (DDC, aromatic L-amino acid decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD) ([[EC Number|EC]] 4.1.1.28) is an approximately 104 kDa protein that belongs to the '''aspartate aminotransferase family''' (fold type 1) of '''[http://en.wikipedia.org/wiki/Pyridoxal_phosphate ''PLP'']-dependent''' (vitamin B6-dependent) enzymes. The catalytically active form of the enzyme exists as a homodimer, typical of this class of enzymes.<ref name="schneider">PMID:10673430 </ref> The homodimeric form of the enzyme purified from [http://en.wikipedia.org/wiki/Sus_scrofa ''sus scrofa''] is shown in complex with the inhibitor '''[http://en.wikipedia.org/wiki/Carbidopa ''carbidopa'']''' to the right. | + | '''DOPA decarboxylase''' ('''DDC, aromatic L-amino acid decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD''') ([[EC Number|EC]] 4.1.1.28) is an approximately 104 kDa protein that belongs to the '''aspartate aminotransferase family''' (fold type 1) of '''[http://en.wikipedia.org/wiki/Pyridoxal_phosphate ''PLP'']-dependent''' (vitamin B6-dependent) enzymes. The catalytically active form of the enzyme exists as a homodimer, typical of this class of enzymes.<ref name="schneider">PMID:10673430 </ref> The homodimeric form of the enzyme purified from [http://en.wikipedia.org/wiki/Sus_scrofa ''sus scrofa''] is shown in complex with the inhibitor '''[http://en.wikipedia.org/wiki/Carbidopa ''carbidopa'']''' to the right. |
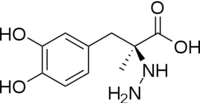
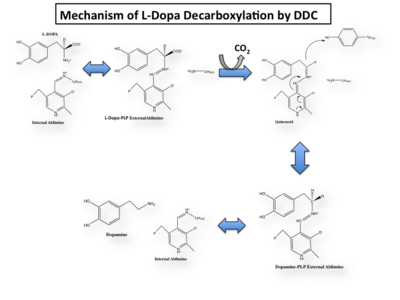
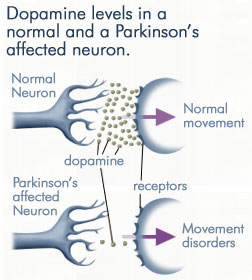
DDC catalyzes the conversion of aromatic amino acids into their corresponding amines. DOPA decarboxylase is responsible for the synthesis of '''[http://en.wikipedia.org/wiki/Dopamine ''dopamine'']''' and [http://en.wikipedia.org/wiki/Serotoninn ''serotonin''] from '''[http://en.wikipedia.org/wiki/L-dopa ''L-DOPA'']''' and [http://en.wikipedia.org/wiki/L-5-Hydroxytryptophan ''L-5-hydroxytryptophan''], respectively. It is highly stereospecific, yet relatively nonspecific in terms of substrate, making it a somewhat uninteresting enzyme to study. Although it is not typically a rate-determining step of dopamine synthesis, the decarboxylation of L-DOPA to dopamine by DDC is the controlling step for individuals with '''[http://en.wikipedia.org/wiki/Parkinson%27s_disease ''Parkinson's disease'']'''<ref name="hadjiiconstantinou">PMID:1904055 </ref> , the second most common neurodegenerative disorder, occuring in 1% of the population over the age of 65. The loss of dopaminergic neurons is the main cause of cognitive impairment and tremors observed in patients with the disease. The hallmark of the disease is the formation of [http://en.wikipedia.org/wiki/alpha-synuclein ''alpha-synuclein''] containing [http://en.wikipedia.org/wiki/Lewy_body ''Lewy bodies'']. | DDC catalyzes the conversion of aromatic amino acids into their corresponding amines. DOPA decarboxylase is responsible for the synthesis of '''[http://en.wikipedia.org/wiki/Dopamine ''dopamine'']''' and [http://en.wikipedia.org/wiki/Serotoninn ''serotonin''] from '''[http://en.wikipedia.org/wiki/L-dopa ''L-DOPA'']''' and [http://en.wikipedia.org/wiki/L-5-Hydroxytryptophan ''L-5-hydroxytryptophan''], respectively. It is highly stereospecific, yet relatively nonspecific in terms of substrate, making it a somewhat uninteresting enzyme to study. Although it is not typically a rate-determining step of dopamine synthesis, the decarboxylation of L-DOPA to dopamine by DDC is the controlling step for individuals with '''[http://en.wikipedia.org/wiki/Parkinson%27s_disease ''Parkinson's disease'']'''<ref name="hadjiiconstantinou">PMID:1904055 </ref> , the second most common neurodegenerative disorder, occuring in 1% of the population over the age of 65. The loss of dopaminergic neurons is the main cause of cognitive impairment and tremors observed in patients with the disease. The hallmark of the disease is the formation of [http://en.wikipedia.org/wiki/alpha-synuclein ''alpha-synuclein''] containing [http://en.wikipedia.org/wiki/Lewy_body ''Lewy bodies'']. | ||
| Line 76: | Line 76: | ||
{{Clear}} | {{Clear}} | ||
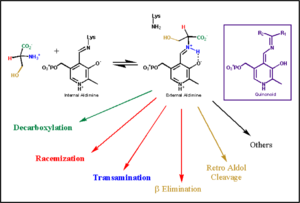
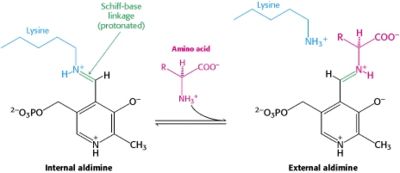
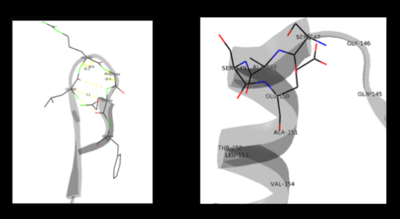
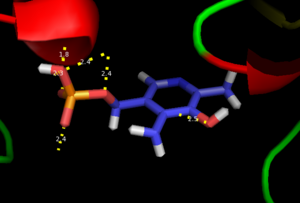
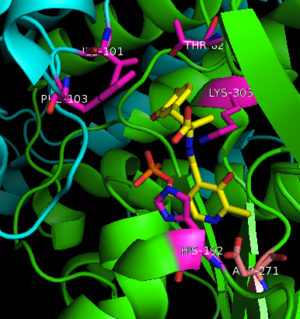
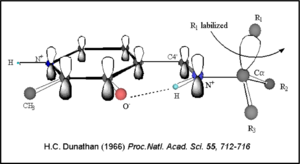
This way, the developing p orbital is aligned for maximal overlap with the extended p system, lowering the energy of the transition state and increasing the rate of the reaction. As well, by controlling substrate orientation, the enzyme can distinguish between '''deprotonation''' and '''decarboxylation'''. | This way, the developing p orbital is aligned for maximal overlap with the extended p system, lowering the energy of the transition state and increasing the rate of the reaction. As well, by controlling substrate orientation, the enzyme can distinguish between '''deprotonation''' and '''decarboxylation'''. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Mechanism Breakdown=== | ===Mechanism Breakdown=== | ||
| Line 102: | Line 97: | ||
With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; whereas, direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier<ref name=Burkhard>PMID: 11685243 </ref>. Even still, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly decarboxylated to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and reducing the side effects of dopamine-rich blood. | With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; whereas, direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier<ref name=Burkhard>PMID: 11685243 </ref>. Even still, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly decarboxylated to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and reducing the side effects of dopamine-rich blood. | ||
| - | |||
| - | |||
==Classification== | ==Classification== | ||
---- | ---- | ||
| Line 141: | Line 134: | ||
[[6eew]] – MpDDC + Trp – Madagascar periwinkle<br /> | [[6eew]] – MpDDC + Trp – Madagascar periwinkle<br /> | ||
[[6eem]] – MpDDC + Tyr<br /> | [[6eem]] – MpDDC + Tyr<br /> | ||
| + | [[6liu]] – poDDC - poppy<br /> | ||
| + | [[6liv]] – poDDC + PLP derivative<br /> | ||
==References== | ==References== | ||
Revision as of 06:47, 8 July 2021
| |||||||||||
3D structures of DOPA decarboxylase
Updated on 08-July-2021
3rbf, 3rbl – hDDC – human
3rch – hDDC + vitamin B6 phosphate + pyridoxal phosphate
3k40 – DDC – Drosophila melanogaster
1js3 – pDDC + inhibitor – pig
1js6 - pDDC
6eew – MpDDC + Trp – Madagascar periwinkle
6eem – MpDDC + Tyr
6liu – poDDC - poppy
6liv – poDDC + PLP derivative
References
- ↑ 1.0 1.1 Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000 Jan 15;8(1):R1-6. PMID:10673430
- ↑ Miles EW. The tryptophan synthase alpha 2 beta 2 complex. Cleavage of a flexible loop in the alpha subunit alters allosteric properties. J Biol Chem. 1991 Jun 15;266(17):10715-8. PMID:1904055
- ↑ Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
- ↑ Miles EW. The tryptophan synthase alpha 2 beta 2 complex. Cleavage of a flexible loop in the alpha subunit alters allosteric properties. J Biol Chem. 1991 Jun 15;266(17):10715-8. PMID:1904055
- ↑ Percudani R, Peracchi A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003 Sep;4(9):850-4. PMID:12949584 doi:http://dx.doi.org/10.1038/sj.embor.embor914
- ↑ Maras B, Dominici P, Barra D, Bossa F, Voltattorni CB. Pig kidney 3,4-dihydroxyphenylalanine (dopa) decarboxylase. Primary structure and relationships to other amino acid decarboxylases. Eur J Biochem. 1991 Oct 15;201(2):385-91. PMID:1935935
- ↑ Aurora R, Rose GD. Helix capping. Protein Sci. 1998 Jan;7(1):21-38. PMID:9514257 doi:10.1002/pro.5560070103
- ↑ Jansonius JN. Structure, evolution and action of vitamin B6-dependent enzymes. Curr Opin Struct Biol. 1998 Dec;8(6):759-69. PMID:9914259
- ↑ 9.0 9.1 Ishii S, Mizuguchi H, Nishino J, Hayashi H, Kagamiyama H. Functionally important residues of aromatic L-amino acid decarboxylase probed by sequence alignment and site-directed mutagenesis. J Biochem. 1996 Aug;120(2):369-76. PMID:8889823
- ↑ Hiscott JB, Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. I. In permissive cells. J Virol. 1979 May;30(2):590-9. PMID:224217
- ↑ Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
Proteopedia Page Contributors and Editors (what is this?)
Brittany Todd, Michal Harel, David Canner, Alexander Berchansky, Brian Hernandez